
PHERAstar FSX
Powerful and most sensitive HTS plate reader
Receptor-ligand kinetics is the study of the rates at which receptors and ligands interact, bind and dissociate. Learn why these types of measurements are important and how to measure them.
 Dr Barry Whyte
Dr Barry Whyte
Barry Whyte is Application Scientist and Science Writer at BMG LABTECH in the United States. He has PhD and Bachelor of Science (BSc) degrees in biochemistry from the University of Bristol in the United Kingdom and more than 20 years of experience in the life sciences and science communications. Over the years, Barry has worked on three continents and traveled widely. He enjoys building on his international work experience and learning new ways to help scientists advance their research.
Receptor-ligand interactions are molecular binding events that alter receptor activity or trigger a response in the cell. Researchers are interested in studying receptor-ligand interactions to understand how cells communicate with their environment and how they operate in response to cell signaling. Receptor-ligand interactions are also an important target for drug discovery (Fig. 1) due to their pivotal role in cell biology.1-4 Significantly, receptors are one of the largest classes of drug targets in biology and G protein-coupled receptors alone represent more than 30% of FDA-approved drugs.5 Understanding the molecular mechanism of these interactions is crucial for optimizing drug design and therapeutic outcomes.
In this overall context, receptor-ligand kinetics provide a handle on the rates at which receptors and drugs interact, bind, and dissociate. Binding kinetics have become a crucial part of the decision-making process in different stages of drug discovery.
Drug-receptor interactions are defined not only by how tightly a drug binds to a receptor (the binding affinity) but by how quickly a drug associates with the target and how long the receptor and drug remain together (binding kinetics). As drug discovery has progressed, this kinetic perspective has grown in importance.
In this blog we look at receptor-ligand kinetics and how they contribute to research and drug discovery. We also look at the benefits offered by a microplate reader to investigate receptor-ligand kinetics and provide some examples of specific applications. The different detection options for receptor-ligand kinetics are highlighted.
Many different types of receptors are found in cells. In the blog Receptor-ligand interactions you can find an overview of some of the main receptor types, how they function, as well as their different locations in the cell. In brief, receptors are widely studied by researchers due to their important role in cell signaling. Many receptors like GPCRs, ion channels, nuclear receptors and receptor tyrosine kinases are also valuable drug targets since they are often control points for cell behavior and act at the interface between signaling and cellular responses.
Receptor-ligand kinetics refers to the study of the rates at which receptors and ligands interact, bind, and dissociate. Receptor-ligand kinetics provide insights into the strength and duration of these interactions, which are crucial for understanding cellular signaling and drug efficacy.
The primary parameters include the association rate constant (kon, also known as the on rate or on rate constant), dissociation rate constant (koff, also referred to as the dissociation rate constant), equilibrium dissociation constant, and binding affinity (KD). Both the on-rate constant (kon) and dissociation rate constant (koff) are important parameters in receptor-ligand kinetics, as they directly influence drug efficacy, receptor occupancy, and therapeutic effectiveness in vivo.
Molecular interactions are dynamic and the binding affinity of a drug for a target receptor depends on the speed of binding (association; termed kon or on rate) and dissociation (known as koff or dissociation rate constant) and on its occupation or residence time (1/koff). These parameters define the binding kinetics of a drug. The binding affinity (Ka) is defined as the ratio of the on-rate to the off-rate, and the binding constant is a special case of the equilibrium constant for receptor-ligand binding. According to the law of mass action, the affinity and kinetics of receptor-ligand binding are governed by the concentrations of reactants and the rate constants, reflecting the physical and thermodynamic factors affecting these interactions.
The purpose of studying binding kinetics is to understand how a particular drug’s kon, koff rates and occupancy influence its interactions – and thus its overall therapeutic utility.6 The relationship between kon, koff and KD is shown in equation 1 where [B], [L] and [BL] indicate concentrations of biomolecule, ligand and biomolecule-ligand complex at equilibrium, respectively. In our case, the biomolecule is a receptor:
 The main method to determine the association rate constant (kon) and dissociation rate constant (koff) is through kinetic binding experiments that measure association and dissociation over time. The observed association rate constant (kobs) describes the observed rate at which a ligand and receptor associate to form a ligand–receptor complex and is dependent on the ligand concentration.
The main method to determine the association rate constant (kon) and dissociation rate constant (koff) is through kinetic binding experiments that measure association and dissociation over time. The observed association rate constant (kobs) describes the observed rate at which a ligand and receptor associate to form a ligand–receptor complex and is dependent on the ligand concentration.
The main goal of receptor-ligand kinetics is to determine the concentrations of various kinetic species at all times based on initial concentrations and rate constants. The kinetics of ligand binding to a receptor can be described using mathematical models that account for the association and dissociation of the ligand-receptor complex.
High affinity of a drug for its receptor can be due to either a high kon or a low koff rate. These parameters should therefore be determined using binding kinetics to understand fully the behavior of a molecule. In kinetic experiments, it is important to consider the concentration of free ligand, which refers to the unbound ligand in solution that has not yet associated with the receptor, as this is critical for accurately determining binding parameters such as affinity and rate constants. These kinetic parameters are ideally determined during the drug discovery stage to save time and money by decreasing failure rates downstream in the pipeline. In many cases, high-throughput methods are needed to profile kinetically hundreds and thousands of compounds.
As we saw earlier, the on-rate constant (kon) and dissociation rate constant (koff) are important parameters in determining drug efficacy and receptor occupancy. The association rate constant (kon) has gained attention as another parameter affecting in vivo efficacy. An optimal receptor residence time is important for efficacy, selectivity, and dosing schedule, and provides more information than affinity alone. Residence time has turned out to be a valuable parameter for researchers focusing on drug development as a good predictor of in vivo efficacy. The involvement of residence time as a predictor of in vivo drug efficacy and the duration of action is well-established.
Thus receptor-ligand binding kinetics provide insight on the duration of the pharmacological effect, in vivo efficacy, and functional selectivity even when equilibrium affinity does not exist (KD, IC50). This is why kinetic assays are a standard in lead optimization workflows. Receptor-ligand kinetics help to translate in vitro data to in vivo performance.
A more detailed introduction to understand how kinetic binding studies across the drug discovery pipeline contribute to the pharmacology of drugs can be seen in the scientific talk Kinetics in Drug Discovery by Martin Redhead.

How do you measure these parameters in practice? Here we give a few examples of receptor-ligand kinetics applications for GPCRs measured on microplate readers. While there are many other types of receptors, these examples give a sense of the possibilities and we will look in a little closer detail here.
In the application note AN335 Analyze binding kinetics with HTRF, the PHERAstar FSX was used to perform kinetic Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) measurements for the binding of Spiperone-d2 to the dopamine receptor D2. The dopamine receptor D2 is a GPCR involved in cell signaling and thought to play a role in several neurological disorders. It is an active target for drug development due to its physiological functions and link to different diseases.
As was mentioned earlier, two of the primary parameters for receptor-ligand kinetics are the on-rate constant (kon), which characterizes the forward rate of complex formation, and the dissociation rate constant (koff). In this study, enzyme kinetic measurements were made to determine the koff and kon rates for the rate of Spiperone-d2 binding to the receptor (Fig. 2). Homogeneous Time-Resolved Fluorescence, which combines Time-Resolved Fluorescence and FRET, was used to determine the kinetic parameters. This type of approach is amenable to high-throughput drug screening on the PHERAstar microplate reader and provides reliable and accurate assessment of the time the ligand engages with the target.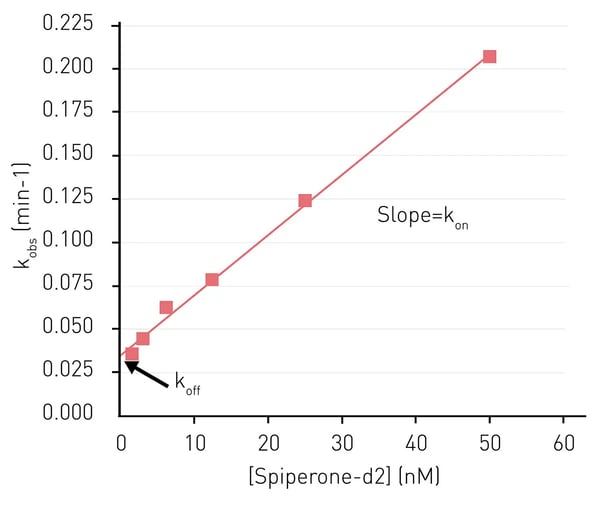
In the scientific talk A TR-FRET approach to measure the kinetics of ligand-receptor binding and its application of fragment screening Professor Steven Charlton discusses a TR-FRET kinetic approach to simultaneously detect the kinetics of multiple compounds. This is demonstrated by a description of a dopamine D2 receptor fragment screening campaign.

Competition assays are used in kinetic studies to determine if two or more molecules bind to the same site and allow binding affinity to be determined. They are useful assays to determine receptor-ligand kinetics. A labeled ligand that is known to bind to a target is incubated with that target in the presence of unlabeled ligand. If the competing ligand binds to the same site, it will displace the labeled ligand in a concentration-dependent manner. Comparing the binding of the same ligand under different conditions can reveal differences in binding affinity and receptor occupancy. Additionally, endogenous ligands present in biological samples can compete with labeled or unlabeled ligands, potentially biasing measurements of receptor density and affinity, so this competition must be accounted for in assay design and interpretation.
In the application note AN386 Competition assay using Fluorescence Polarisation to determine the Residence Times for Calcitonin and AMYR agonist, AM833 competition assays were carried out on the PHERAstar® FSX to study GPCRs. The type of approach described in this application note is beneficial since it avoids the need to label the receptor. The AMYR agonist AM833 used in this study represents a promising novel alternative for the treatment of obesity.
Amylin and calcitonin are peptide hormones that act through GPCRs. The high sensitivity in fluorescence polarization and simultaneous dual emission of the PHERAstar FSX allowed for very fast kinetic measurements. The use of fluorescence polarization for Kon and Koff rate determination in competition assays represents a useful alternative strategy to existing methods for evaluating agonists and antagonists of GPCRs (Fig. 3).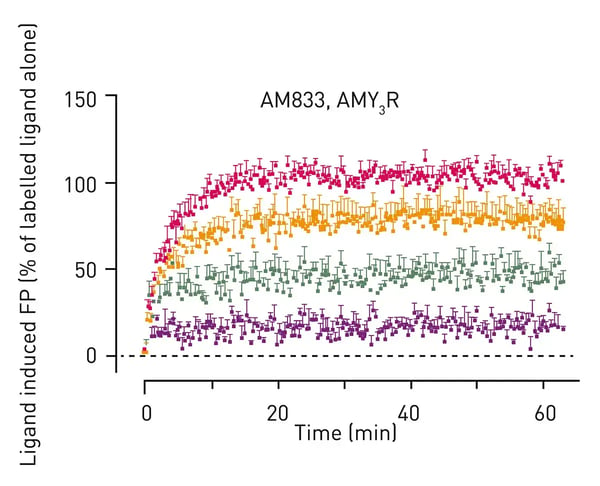
Sometimes when making kinetic measurements for receptor-ligand interactions it is advantageous to measure the changing levels of two molecules or signals in parallel. Cell signaling events are complex, dynamic processes and the ability to measure two molecules in real time can give useful information on the most relevant interactions for a particular event.
In the application note AN410 Multiplexing the production of two G protein-coupled receptor second messengers using spectrally resolved fluorescent dyes
two fluorescent dyes were used to follow two signaling events (calcium and potassium flux) downstream of the GPCR M3 muscarinic acetylcholine receptor and the dopamine D2 receptor. Fluo-GoldTM (a dye for calcium measurements) and Thallos AM (a dye to measure potassium ion flux) were used for simultaneous measurements on the CLARIOstar® Plus (Fig.4). The LVF MonochromatorTM allowed easy adjustment of the detection wavelengths to optimize the measurements.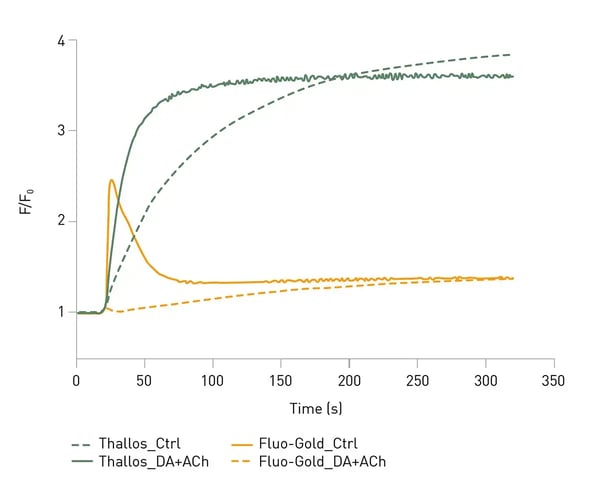
A second example of multiplexing is provided in the application note Simultaneous detection of GPCR second messengers in living cells. In this case, the CLARIOstar was used to quantify second messengers for GPCR signaling in live cells using biosensors for diacylglycerol, phosphatidylinositol 4,5-bisphosphate (PIP2), and calcium. Highly sensitive detection of genetically engineered sensors for measurements of second messengers were possible using this approach. Spectrally resolved variants indicated simultaneous changes in these second messengers following Gq activation. Examples of the kinetic measurements for diacylglycerol and calcium are shown in Fig. 5.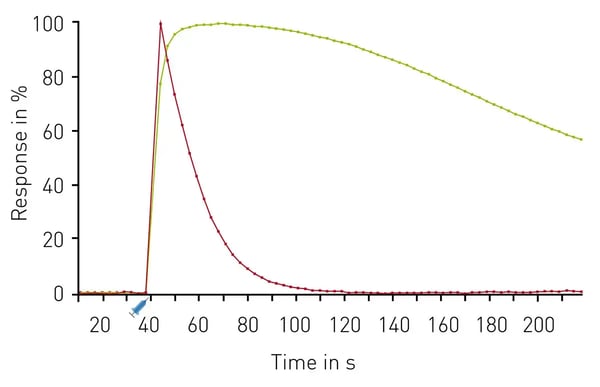
The applications highlighted earlier for the different receptors reflect some of the many binding assays available to study receptor-ligand kinetics with plate readers. Scientists have a range of detection technologies available to study the kinetics of these interactions but they are typically fluorescence- or luminescence-based assays for cell-based or biochemical receptor assays. The detection options include Förster´s Resonance Energy Transfer (FRET), TR-FRET and luminescence assays like Bioluminescent Resonance Energy Transfer (BRET, NanoBRET).
As we saw in the previous examples, receptor-ligand kinetics can be measured by monitoring the real-time signal after adding the receptor ligand to look at the association reaction or using excess of an unlabeled competitor to look at signal decay in the dissociation phase. By measuring receptor-ligand kinetics in live cells you can get information that is physiologically relevant to a particular receptor. These assays can be suitable for higher throughput and for screening compound libraries. Functional coupling is possible and binding kinetics for GPCRs for example can be linked to cAMP production, beta-arrestin recruitment or calcium flux. These measurements are indirect by design and reflect the combined behavior of drug binding and probe behavior but these types of assays are immensely useful with carefully designed controls.
We have already highlighted some of the technological features of a microplate reader that facilitate the study of receptor-ligand interactions in the blog Receptor-ligand interactions The features relevant to receptor-ligand kinetics studies are summarized in Table 1.
Table 1. Summary of benefits offered by microplate readers for receptor-ligand kinetics
| Feature | Benefit | Note |
| Simultaneous Dual Emission | Halves read time and improves data quality for all dual emission assays, such as FRET, TR-FRET, fluorescence polarization, and BRET, as the reader can detect two emission wavelengths in a single measurement. | Available on the PHERAstar FSX |
| Enhanced Dynamic Range | Ensures accurate signal quantification across low to high concentrations of ligands and targets without running into the risk of signal saturation | Available on the PHERAstar FSX, VANTAstar and the CLARIOstar Plus |
| Sampling rate | Sampling rate of 100 data points per second. Particularly useful for resolving fast kinetic processes and for generating higher density datasets of interaction events | Sampling rates for PHERAstar FSX and the CLARIOstar Plus |
| TRF laser | With 60 laser flashes per second, it allows ultra-fast TR-FRET kinetic measurements, and even “flying mode” detection | Available on the PHERAstar FSX |
| Injectors | Injection and simultaneous detection are useful for fast reactions and kinetic measurements. Significant asset for reading in kinetic well mode since they offer the best performance in terms of speed and throughput (sampling rate) | All BMG LABTECH microplate readers come with up to two injectors per reader and are compatible with plate formats up to 384 wells |
| Range of throughput options and automation | Accelerates measurements and is particularly useful for screening campaigns. Support for 96-, 384-, 1536- and 3456-well microplate formats | BMG LABTECH readers offer excellent robotic integration capabilities, multi-user control, digital signature and FDA 21 CFR part 11 compliance. Robotic software interface permits easy integration into all leading robotic platforms. |
| Atmospheric Control Unit | Independently control oxygen and carbon dioxide concentrations for cell-based assays (as low as 0.1% oxygen on the CLARIOstar Plus) | Ideal selection for plate and well-mode kinetic measurements that require specific atmospheric conditions and rapid sampling |
| Advanced Assay Stability (AAS) | Maintains constant temperatures between 18 and 45°C by automatically heating or cooling the measurement chamber. Prevents environmental fluctuations from affecting assay performance, enabling consistent conditions throughout entire batches or screening campaigns | Available on the PHERAstar FSX |
The number of studies using receptor-ligand kinetics assays on BMG LABTECH microplate readers continues to expand. These studies are benefitting from more options for advanced kinetic analysis, continuous/real-time measurements as well as label-free detection. These investigations are also being strengthened by increased adoption of live cell and native-context assays as well as ongoing improvements to in vitro assays.
A broader overview of how advanced assays drive preclinical drug discovery is given by Nick Holliday in his testimonial Pioneering Pre-Clinical Drug Discovery with Advanced Assays.

Enhancements to data analysis capabilities are aiding basic research and helping to improve the discovery and development of new drugs. Advances in molecular pharmacology and detailed analyses of ligand-receptor binding kinetics are providing deeper insights into drug action and receptor behavior, supporting a better understanding of molecular mechanisms that influence drug efficacy and receptor signaling. In addition, improved high throughput discovery workflows are advancing all the time for the different steps of early drug discovery.
With these improvements come efficiencies, improved performance, and better use of resources.
Whatever your requirements, BMG LABTECH has the microplate reader for your receptor-ligand kinetics assay whether you are interested in cell signaling, drug discovery or another application.
The PHERAstar FSX was specifically conceived for screening campaigns and is your go-to reader for the fastest high-performance high-throughput screenings.
Both the VANTAstar® and CLARIOstar Plus allow for wavelength flexibility and include Enhanced Dynamic Range technology for superior performance in a single run of endpoint and kinetic mode assays. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options for endpoint and kinetic mode assays.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities for endpoint and kinetic mode assays. In addition, the Omega series, CLARIOstarPlus, and PHERAstar FSX microplate readers can be equipped with on-board injectors that can offer the very best options for detection at the time of injection for endpoint and kinetic mode assays.
Collectively, BMG LABTECH multi-mode readers combine high-quality measurements with miniaturized assays, short measurement times, and offer considerable savings on materials and other resources for endpoint and kinetic mode assays.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Flexible microplate reader with simplified workflows
Receptor-ligand interactions are crucial for cell signalling. They are also important for drug discovery. How do microplate readers deliver benefits to both?
Redox processes play an important role in cell homeostasis. Read here, how to monitor cellular redox changes with roGFP using the most sensitive, high-performance plate readers from BMG LABTECH.