Introduction
There is increasing evidence that targeting the amylin receptors, may improve weight control. AM833, a lipidated peptide analogue of Amylin and a non-selective agonist of Amylin and Calcitonin G-Protein Coupled Receptors (GPCR’s), is a potential drug that could be used for this purpose. The peptide is currently undergoing phase II clinical trials for the treatment of obesity.1
In this study, the residence times (1/Koff) of AM833, at the Calcitonin receptor (CTR) and Amylin receptors, AMY1R and AMY3R, were evaluated. Residence time is the time taken for 50% of a ligand to dissociate from a receptor and relies on the determination of the association and dissociation rate constants Kon and Koff for the receptor/drug interaction.2 One method for determination of Kon and Koff rates is a competition assay between a fluorescently labelled ligand with known Kon and Koff rates and the unlabelled ligand of interest (e.g. AM833) for a particular GPCR.3 To generate the signal for agonist/receptor interaction, FRET (fluorescence resonance energy transfer) and BRET (bioluminescence resonance energy transfer) techniques have been used for competition assays in microplate readers. However, these competition assays require both a labelled agonist and labelled GPCR.3
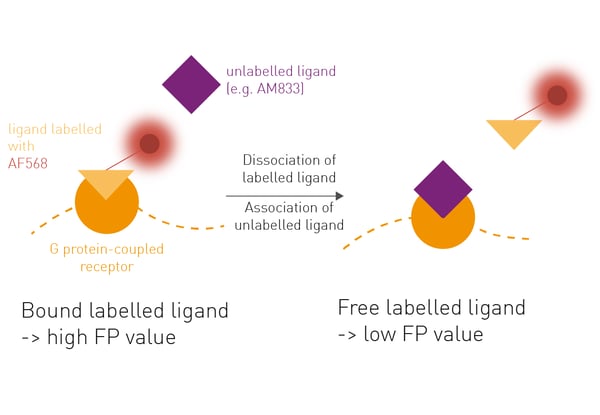
Here we describe the use of fluorescence polarization (FP) to determine agonist/receptor Kon and Koff rates in a kinetic competition assay, with the advantage that labelling of the receptor is unnecessary.
Assay principle
FP measures the rotation of labelled molecules in solution. Larger molecules rotate slower than smaller molecules. The slower they rotate the higher the FP signal. In this competition assay, the salmon-calcitonin peptide sCT(8-32) labelled with Alexafluor568 was used as tracer with known Kon and Koff rates at the tested receptors. The competition assay is then performed by adding membranes containing test receptors to a mixture of a fixed concentration of tracer and varying concentrations of the unlabelled ligand of interest (e.g. AM833). Upon binding of the unlabelled ligand and displacement of the tracer, FP values of the competition assay will decrease.- 96-well plates, black, PP, round bottom (Corning)
- HEK293F cells transfected with CTR, AMY1R, or AMY3R (Cells and LipofectAMINE 2000 kit from Thermo Fisher)
- salmon-calcitonin peptide sCT(8-32) labelled with Alexafluor568
- unlabelled AM833
- PHERAstar microplate reader (BMG LABTECH)
Membrane-Based Kinetic binding competition assay
24 h after transfection with sequences for the different receptors, HEK293F cells were harvested and plasma membranes were prepared by sucrose density centrifugation.
The competition assays to determine ligand binding were performed in HBSS + 10 mM HEPES + 0.1% ovalbumin (pH 7.4) in each well of a black 96-well plate, containing 10 nM tracer, with or without unlabelled competitor. Control wells containing 1 mM of unlabelled sCT and 10 nM sCT8-32:AF568 were used to define unspecific binding. Competition assays were read on a PHERAstar plate reader with the following settings:
Instrument settings
|
Optic settings
|
Fluorescence Polarization, plate mode kinetic
|
|
|
Optic module
|
FP 560 615 615 |
|
| General settings |
Number of flashes | 100 |
| Settling time | 0.5 sec | |
|
Kinetic settings
|
Number of cycles
|
200
|
|
Cycle time
|
19 sec
|
|
|
Incubation
|
30 °C
|
|
After baseline acquisition, membranes were added, and data of the competition assay was collected for 60 minutes. Graphs of the kinetic response were generated by normalising the means of individual experiments to 100% based on the last time point from wells without added competitor.
The Kon and Koff rates for unlabelled ligand were determined by fitting the kinetic data of the competition assay to the Motulsky-Mahan rate equation.4
Results & Discussion
Typical kinetic traces that were obtained from FP experiments are shown in figure 2.
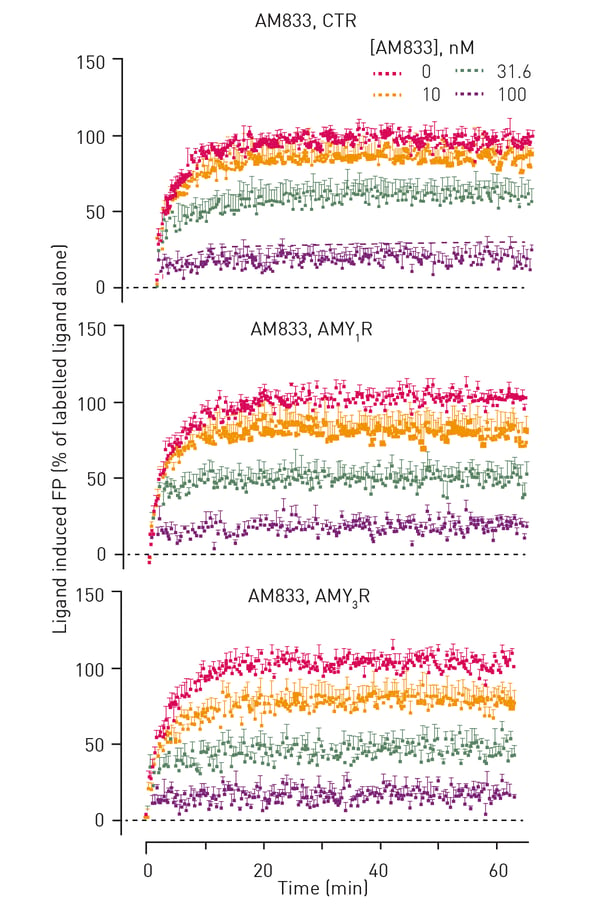 Apart from AM833, residence times were also determined for six other known peptide agonists of calcitonin receptors (table 1). As can be seen, AM833 exhibited rapid dissociation with a residence time (∼3–6 minutes) that was not different between receptor subtypes. AM833 exhibited a short residence time at CT family receptors that was similar to pramlintide, human calcitonin (hCT) and rat-amylin (rAmy) and amylin analog AM1213. In contrast, AM1784 and sCT had a slower off-rate and longer receptor residency times.
Apart from AM833, residence times were also determined for six other known peptide agonists of calcitonin receptors (table 1). As can be seen, AM833 exhibited rapid dissociation with a residence time (∼3–6 minutes) that was not different between receptor subtypes. AM833 exhibited a short residence time at CT family receptors that was similar to pramlintide, human calcitonin (hCT) and rat-amylin (rAmy) and amylin analog AM1213. In contrast, AM1784 and sCT had a slower off-rate and longer receptor residency times.
Tab. 1: Mean residency times for agonists at sCT, AMY1R and AMY3R receptors
| CTR Mean T1/2 (min) | AMY1R Mean T1/2 (min) | AMY3R Mean T1/2 (min) | |
| AM833 | 3.22 | 5.40 | 3.64 |
| Pramlintide | 1.37 | 1.56 | 2.02 |
| sCT | 47 | 45 | 63 |
| hCT | 1.71 | 1.15 | 1.96 |
| rAmy | 1.13 | 1.39 | 2.80 |
| AM1213 | 1.55 | 1.40 | 2.74 |
| AM1784 | 1.73 | 291 | 652 |
sCT showed a slow off-rate from CT family receptors, which resulted in a long residency time and prolonged duration of action in vitro and in vivo.
However, despite no causal cellular mechanism having been identifi ed, sCT–containing compounds have been associated with tumour growth and sCT is restricted from long term clinical use because of an unfavourable risk-benefit profile.1 The long residency time for sCT might be a contributing factor to these side effects and compounds such as AM833 with shorter residency times might prove to be better drugs for the treatment of obesity.
Conclusion
The use of FP for Kon and Koff rate determination in competition assays represents a useful alternative strategy to existing methods for evaluating agonists and antagonists of GPCR’s. In addition, the PHERAstar plate reader is perfectly equipped for these kinetic experiments. The instrument features high sensitivity in FP, luminescence and fluorescence with simultaneous dual emission readings, allowing very fast kinetics for many applications.
References
-
AM833 Is a Novel Agonist of Calcitonin Family G Protein–Coupled Receptors: Pharmacological Comparison with Six Selective and Nonselective Agonists; Madeleine M. Fletcher, Peter Keov, Tin T. Truong, Grace Mennen, Caroline A. Hick,Peishen Zhao, Sebastian G.B. Furness, Thomas Kruse, Trine R. Clausen, Denise Wootten, and Patrick M. Sexton: J Pharmacol Exp Ther 377:417–440, June 2021.
-
Drug-Target Residence Time Affects in Vivo Target Occupancy through Multiple Pathways; Kin Sing Stephen Lee, Jun Yang, Jun Niu, Connie J. Ng, Karen M. Wagner, Hua Dong, Sean D. Kodani, Debin Wan, Christophe Morisseau, and Bruce D. Hammock: ACS Central Science 2019 5 (9), 1614-1624 DOI: 10.1021/acscentsci.9b00770
-
Binding kinetics of ligands acting at GPCRs: David A. Sykes, Leigh A. Stoddart, Laura E. Kilpatrick, Stephen J. Hill; Molecular and Cellular Endocrinology 485 (2019) 9–19.
-
Motulsky HJ and Mahan LC (1984) The kinetics of competitive radioligand binding predicted by the law of mass action. Mol Pharmacol 25:1–9