Assays empower biomedical research especially drug discovery
Assay development is needed to qualitatively assess a compound or examine a compound’s effects. Whether the effect of a drug is correctly characterised, or the course of a patient's disease can be correctly diagnosed, depends primarily on the validity of the assays used. It is therefore important to proceed with the utmost care in the development and selection of all assays.
An assay is an investigative procedure used in pharmacology, diagnostic medicine, environmental biology or molecular biology, to measure the presence, quantity or activity of a target entity. The analyte can thereby be a drug, a compound but also an enzyme, a cell or a whole organism. A bioassay is a combination of science and statistical methods to determine (relative) potency.
Such assays exist in very different types from antibody-based assays such as ELISAs, enzyme activity assays, reporter assays, protein/protein interaction or nucleic acid quantification assays up to sophisticated assays such as single-molecule activation assays in drug screening.
Failure in the correct development of a bioassay will cause problems e.g., in the approval of drugs or may completely prevent licensure as high-quality standards are required here. Most of the problems associated with bioassay-related quality standards can be avoided with correct development, qualification, validation and control of the reference standard.
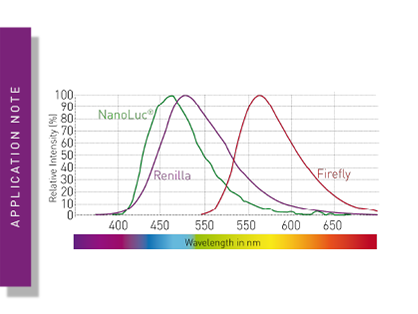
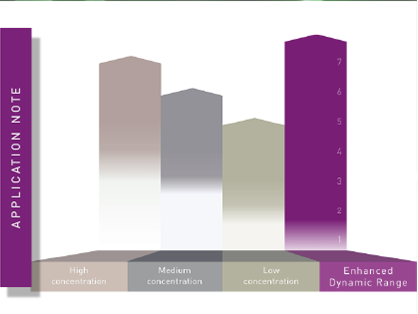
BMG LABTECH microplate readers represent an ideal platform to address key steps in assay development. Equipped with features like temperature incubation and CO2 and O2 gas control, and able to shake and even add reagents via the injector system, microplate readers can also be used for multiple steps in the workflow of the assay procedure, instead of being used only for measurement. This enables holistic monitoring of the entire assay procedure under realistic conditions, significantly reducing potential sources of error during assay development. Multi-mode readers also allow the direct comparison and easy switching between different detection modes during assay development. Thanks to BMG LABTECH’s patented LVF Monochromator, users of the CLARIOstar Plus and the VANTAstar enjoy very high flexibility in the selection of assay-specific wavelengths and bandwidths with filter-like sensitivity. This feature is particularly advantageous for assay development, as measurement settings, such as the ideal measurement wavelength, are usually not pre-determined and may vary among different assays. The same applies to signal intensities, which are often difficult to predict during development. With the Enhanced Dynamic Range feature, very dim and very bright signals can be detected automatically with the optimal settings.
Automation is an important prerequisite when high-throughput analyses are required. Microplate readers from BMG LABTECH can easily be integrated in automated systems. Small footprint, multiple robotic software interfaces and an automation-friendly plate carrier guarantee an easy integration into all leading robotic platforms. BMG LABTECH's analysis software MARS simplifies and automates recurring data analysis and interpretation, which is a great support especially for large throughputs.