
SPECTROstar Nano
Absorbance plate reader with cuvette port
ELISAs are a popular tool to detect or measure biological molecules in the life sciences. Find out how microplate readers can be used to advance research using immunoassays.
 Dr Barry Whyte
Dr Barry Whyte
Enzyme-Linked Immunosorbent Assays (ELISAs) are an essential technique in today’s laboratory with many applications in the life sciences. They are often the method of choice to detect or measure specific biological molecules (analytes) for diagnostics, drug discovery or fundamental research. In this blog, we look at immunoassays over the years, provide some examples of current and emerging applications, discuss some tips for optimization, and look at some of the benefits of the different ELISA options available to researchers.
ELISAs were first developed in the mid-1970s independently by two research groups. Peter Perlman and Eva Engvall at Stockholm University in Sweden developed an ELISA method as a tool for quantifying soluble antigens which was published in 1971.1,2 Anton Schuurs and Bauke van Weemen at the research laboratories of NV Organon, Oss, the Netherlands developed a similar technique which was also published in 1971.1,3 Both groups made significant contributions to the initial development of ELISAs, a method which has gone on to be a cornerstone of many applications in immunology, clinical diagnostics, and biomedical research.
The use of ELISAs has flourished in the life sciences. This is reflected in scientific discoveries across many research disciplines including immunology, molecular biology, medicine, and diagnostics. Specific areas where they have had a wide impact include virology, bacteriology, biotechnology, fungal biology, cancer and oncology (e.g. biomarker discovery), blood typing, autoimmune diseases, food science (including allergy testing), infectious disease surveillance, drug discovery and development as well as vaccine development.
ELISAs played a pivotal role in the early detection of the human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS). The development of ELISA-based tests for detecting HIV antibodies revolutionized HIV screening and diagnosis, which enabled the detection of infected individuals and the introduction of preventive measures.
ELISA assays are extensively used for the detection, surveillance, and monitoring of a wide range of other infectious diseases including malaria, tuberculosis, dengue fever and influenza. The ability of ELISA assays to detect pathogen-specific antigens or antibodies facilitates disease diagnosis, epidemiological studies, and public health interventions.
In drug development and discovery, ELISAs are useful to quantify drug concentrations in biological samples where they find applications in studies of drug efficacy, pharmacokinetics, and pharmacodynamics. This type of application helps guide the development and optimization of therapeutics. ELISAs can also be used to measure the antibody responses elicited by vaccines. For example, scientists can use ELISA-based tests to assess the immunogenicity of a new vaccine, determine antibody levels in vaccinated individuals, and to optimize vaccine formulations to enhance efficacy.
In research, diagnostics, the manufacture of biologicals and other activities, it is critical to verify test results and confirm findings. Quality control is therefore another area that benefits from ELISA measurements. From food safety to the manufacture of new drugs, ELISAs provide valuable checkpoints and assist in decision making.
Collectively, ELISAs have supported a wide range of applications for more than 50 years of research in the life sciences. They continue to be developed for new uses spurred by further advances in detection technologies and diagnostics.
A good starting point when considering different ELISA assays is the sandwich ELISA which comprises an antibody-coated plate and a screening antigen (Fig.1). 4,5
Two antibodies are used that bind to different epitopes of the target antigen. The first, the capture antibody, is the antibody bound to the bottom of the well of a microplate. The capture antibody also binds to one of the antigen epitopes of the analyte to be measured. The second, the detection antibody, is in solution and binds to the second epitope of the target antigen. The second antibody is labelled with a fluorophore or with an enzyme that converts a substrate to a detectable molecule such as a chromophore or lumiphore.
Detection may involve measurement of absorbance or different types of fluorescence or luminescence signals. If more analyte is present in the sample, increasing amounts of detection antibody and signaling enzyme bind to the analyte. This corresponds to more converted substrate and a higher signal intensity that correlates with the concentration of the analyte.
There are several ways to distinguish one type of ELISA from another. In addition to the ubiquitous sandwich assay, three other types of ELISA assays are widely recognized: direct (antigen-coated plate; screening antibody), indirect (antigen-coated plate; screening antigen/antibody) and competitive (screening antibody). 4
A direct ELISA involves immobilizing an antigen directly on the surface of a microplate well. Detection takes place using a labelled antibody specific to the antigen.
In an indirect ELISA, antigen is immobilized on the surface of a microplate. A primary antibody specific to the antigen binds to the immobilized antigen and a secondary antibody, which is labelled with an enzyme, binds to the primary antibody. The conversion of substrate by the enzyme produces a detectable signal.
A competitive ELISA involves competition between the labeled antigen and unlabeled antigen in the sample for binding to a limited number of immobilized antibodies. The amount of labeled antigen detected is inversely proportional to the mount of unlabeled antigen in the sample.
Over time, researchers have worked hard to increase the speed and reliability of ELISA assays. An important distinction in this respect is the difference between homogeneous and heterogeneous assay types.6
Homogeneous assays refer to a format allowing simple mix-and-measure procedures that remove the necessity to further process samples by separation steps or additional wash steps. Heterogeneous assays include at least one wash step to remove unbound components which is needed to reduce background noise. Homogeneous assays result in a significant reduction of sample handling and overall working steps and often transfer well to high-throughput processes. The shift to homogeneous assays has been facilitated by the integration of more advanced detection technologies into ELISA assays.
Microplate readers offer several key advantages for the performance of ELISAs. First, they are ideally suited to deliver a high-throughput environment where they are typically used with 96-well microplates. Microplate readers are compatible with automated liquid handling systems and robotic platforms, which facilitate high-throughput screening and minimize any errors that might be introduced due to steps like manual pipetting. The simultaneous automated processing of multiple samples or assays in parallel increases throughput and efficiency, which allows for cost-effective solutions to be implemented.
Microplates offer solutions that allow researchers not only to scale up but also to scale down their assays according to experimental needs. They therefore provide flexibility in assay design and optimization whether researchers are screening large compound libraries or performing small-scale experiments.
Today researchers have many microplate reader and detection options at their fingertips for immunoassays. Absorbance is the most frequently used detection mode for ELISAs. However, fluorescence intensity, time-resolved fluorescence and luminescence assays are also used and bring distinct benefits. Here we consider the different detection methods for ELISA assays on microplates and highlight distinctive features of BMG LABTECH solutions.
Changes in absorbance are frequently measured in ELISA measurements. Colorimetric assays rely on the enzymatic conversion of a colourless substrate into a coloured product by an enzyme that is conjugated to the detection antibody. Colorimetric detection is frequently used in ELISAs due to its simplicity, cost-effectiveness, and compatibility with microplate readers. The two most encountered bioconjugated enzymes in colorimetric assays are horseradish peroxidase and alkaline phosphatase (Table 1). Colorimetric substrates include tetramethylbenzidine (TMB) and 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS). An example of the use of these enzymes and substrates in measuring classic ELISA assays is given in the application note Overview of ELISA assays and NADH/NADPH conversion detection.
All BMG LABTECH microplate readers can acquire single or multiple discrete wavelengths in a short period of time. Spectrometer-based microplate readers acquire the whole range of light transmitted through the probe. This makes them ideally suited for colorimetric ELISA assays that are routinely performed in the laboratory. If needed the ultrafast spectrometer can capture an entire spectrum from 220 to 1000 nm in less than 1 second/well.
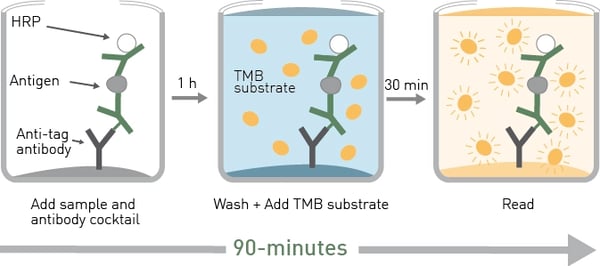
As mentioned earlier, homogeneous assays offer advantages over traditional heterogeneous immunoassays. The SimpleStep ELISA® kits streamline ELISA experiments to a semi-homogeneous format that results in a fast, single-wash process. A typical ELISA involves multiple wash steps that can take 3-5 hours to perform. The use of SimpleStep ELISA® kits reduces the time needed to 90 minutes.
The essential features of the SimpleStep ELISA kit are described in the application note Highly sensitive ELISAs in 90-minutes: SimpleStep ELISA kits and the SPECTROstar® Nano The SimpleStep ELISA technology enables the sandwich ELISA to be performed semi-homogeneously by the addition of an immobilization antibody (Fig.2). This approach reduces assay time considerably but maintains high sensitivity, specificity and reproducibility.
 The use of this type of assay for neuroscience applications is described in the application note Fast and accurate detection of Alzheimer’s Disease targets with SimpleStep ELISA kits and SPECTROstar Nano. Here SimpleStep ELISA kits were used to quantify brain derived neurotrophic factor, tau protein and TREM2 (triggering receptor expressed in myeloid cells 2).
The use of this type of assay for neuroscience applications is described in the application note Fast and accurate detection of Alzheimer’s Disease targets with SimpleStep ELISA kits and SPECTROstar Nano. Here SimpleStep ELISA kits were used to quantify brain derived neurotrophic factor, tau protein and TREM2 (triggering receptor expressed in myeloid cells 2).
The determination of the expression of biomarkers is essential for optimizing immuno-oncology therapies. In the application note Immuno-oncology biomarker measurement with high sensitivity and speed using SimpleStep ELISA kits and SPECTROstar Nano, SimpleStep ELISA kits were used to detect and measure three cancer biomarkers: programmed death ligand 1, interleukin 8, and interferon gamma. The results for human interleukin 8 are shown in Fig.3 and demonstrate that the SimpleStep ELISA kits used on the SPECTROstar Nano provide fast and accurate detection of this predictive immune oncology biomarker over a range of concentrations.
Under the right conditions, fluorescence intensity correlates linearly with the concentration of a molecule to be measured (e.g., analyte coupled to the excited fluorophore) which makes it appropriate for quantitative analyses using ELISAs. Fluorescence offers greater sensitivity than absorbance for measurements and can be carried out on low sample volumes.
In the application note Competitive bead-based fluorescence assay to quantify antibody concentration the CLARIOstar® microplate reader was used in a bead-based assay for the quantification of cell line secreted protein in a high throughput format. The assay uses streptavidin-coated capture beads to which a defined amount of biotinylated protein A is bound. This application is an example of a competitive assay where the fluorescence marker, a fluorescein isothiocyanate (FITC)-labeled human antibody, competes with the analyte for binding to protein A (Fig.4). The more analyte present in the well, the more FITC-labeled antibody is replaced which results in a high fluorescence signal. The competitive assay format is not limited to monoclonal antibody detection but can be extended to a variety of proteins and antibody fragments.
Time-Resolved Fluorescence (TRF) and Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) are fluorescence-based techniques that allow for the analysis of molecular interactions in biological and other processes. TR-FRET is based on TRF measurement and Förster’s Resonance Energy Transfer (FRET) between donor and acceptor molecules. Both TRF and TR-FRET can be used for immunoassays.
TRF is like fluorescence intensity but differs in the timing of the measurements. TRF relies on the use of lanthanides, a type of fluorescent molecule that emits over longer time periods after excitation compared with other standard fluorescent dyes. This delay in emission is helpful in reducing background noise during measurements.
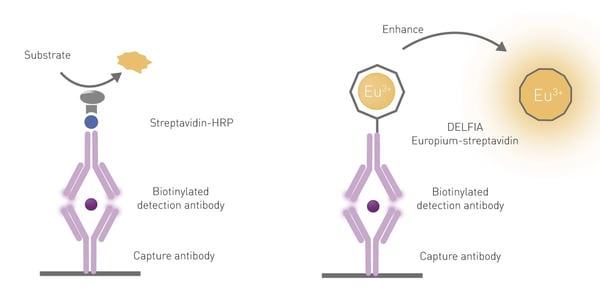
The advantages of using TRF for immunoassays include its sensitivity and its ability for use in multiparameter detection (multiplexing). One of the most used TRF immunoassays is DELFIA® (dissociation-enhanced lanthanide fluorescence immunoassay) and a comparison of a standard ELISA with DELFIA is shown in Fig.5.
DELFIA is a heterogeneous assay and was developed to work on a similar principle to an ELISA. Both use a capture antibody bound to the bottom of the microplate well to bind the analyte. However, to produce a fluorescent signal a dissociative enhancement step is required in DELFIA to free europium molecules from their cage on the antibody (Fig.5). DELFIA assays provide a wide dynamic range and robust stable signals that can be measured months after carrying out an assay. However, they are not suited to high-throughput screening since they involve binding, incubation, and wash steps.
 DELFIA assays can be performed with high sensitivity by TRF on the PHERAstar FSX. This is demonstrated for matched antibody pairs (MAPs) in the application note Time Resolved Fluorescence (TRF) immunoassay in 384-well format using a matched antibody pair kit and the PHERAstar® FSX. Here MAPs were demonstrated to be suitable for use in miniaturized TRF immunoassays and delivered the required sensitivity, specificity, and reproducibility of results. In the application note A DELFIA time-resolved fluorescence cell-mediated cytotoxicity assay performed on the PHERAstar FS estrogen was shown to dramatically reduce cell death induced by NK92 natural killer cells. In this case, DELFIA assays were performed in a 96-well microplate and were shown to be an alternative to the chromium release assay which involves the use of radioactivity.
DELFIA assays can be performed with high sensitivity by TRF on the PHERAstar FSX. This is demonstrated for matched antibody pairs (MAPs) in the application note Time Resolved Fluorescence (TRF) immunoassay in 384-well format using a matched antibody pair kit and the PHERAstar® FSX. Here MAPs were demonstrated to be suitable for use in miniaturized TRF immunoassays and delivered the required sensitivity, specificity, and reproducibility of results. In the application note A DELFIA time-resolved fluorescence cell-mediated cytotoxicity assay performed on the PHERAstar FS estrogen was shown to dramatically reduce cell death induced by NK92 natural killer cells. In this case, DELFIA assays were performed in a 96-well microplate and were shown to be an alternative to the chromium release assay which involves the use of radioactivity.
The advantages of using TR-FRET for immunoassays include ease of use, its sensitivity, as well as its ability to scale through automation.7 As a homogeneous assay, detection by TR-FRET of a donor-acceptor complex does not require physical separation from unbound components. This eliminates the need for separation steps as well as the multiple washing steps needed in other methods.
In the application note Excellent assay performance of THUNDER™ TR-FRET cell-based cytokine assays performed on the PHERAstar FSX examples of immunoassays suitable for drug discovery efforts were validated. A panel of five THUNDER human cytokine assays were tested for their compatibility with use on the PHERAstar FSX and were determined suitable for quantification of cytokines by TR-FRET measurements. The assay principle for these homogeneous assays is shown in Fig.6.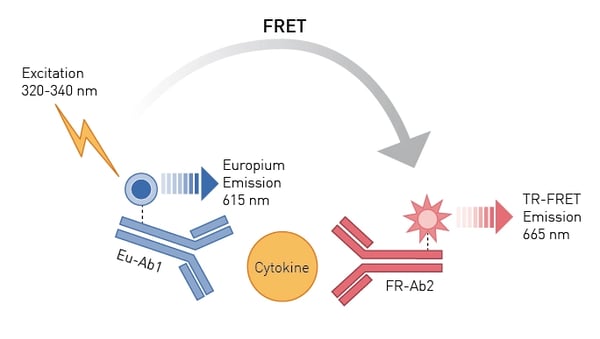 You can learn more about the advantages offered by the PHERAstar FSX and CLARIOstar for THUNDER assays in the following video THUNDER cell signaling assays on our microplate readers:
You can learn more about the advantages offered by the PHERAstar FSX and CLARIOstar for THUNDER assays in the following video THUNDER cell signaling assays on our microplate readers:

The Decay Curve Monitoring (DCM) feature of the PHERAstar FSX is particularly useful for the development of TRF and TR-FRET assays for drug discovery like those described in the THUNDER™ TR-FRET application note. The dedicated photon counting detection system of the PHERAstar FSX enables simultaneous monitoring of both donor and acceptor decay curves, which helps improve signal detection and reduces background noise. Collectively, features that can be optimized like DCM, rapid focal height adjustment, and laser-based excitation are particularly important when miniaturizing to microplates compatible with high-throughput applications for drug discovery and development.
Luminescence detection offers high sensitivity for ELISA measurements. Luminescent immunoassays are available that offer no-wash detection and significant savings in assay time and make for attractive homogeneous assays. Homogeneous assay kits are readily available that allow for detection on luminescence plate readers.
SPARCL (spatial proximity analyte reagent capture luminescence) is an immunoassay that detects analytes in solution and generates a light signal (Fig.7). The immunoassay involves two antibodies: one that binds the chemiluminescent substrate acridan and another that binds horseradish peroxidase. The binding of these antibodies to the analyte brings the substrate and enzyme into proximity. A light signal is produced when the substrate and enzyme are close together upon addition of a trigger solution (H2O2). The signal is proportional to the amount of analyte present in the sample.
In the application note Using SPARCL technology to develop immunoassays for biomarker detection and pharmacokinetic studies the FLUOstar® Omega was used for the detection of interleukin-8 as a representative biomarker. The ability to read at the time of injection for samples on 96-well plates allowed for rapid measurement of the entire luminescent signal.
The SPARCL assay is also highly compatible with the CLARIOstar microplate reader as demonstrated in the application note Verifying SPARCL performance on the CLARIOstar equipped for reading at time of injection. In this study, human IgG levels were measured at the time of injection on 96-well and 384-well microplates. Data for the luminescent output from SPARCL assays performed in 384-well plates are shown in Fig.8.
 The benefits of the enhanced dynamic range of the CLARIOstar for chemiluminescent measurements were demonstrated in the application note Comparing site specific phospho-tau in normal and Alzheimer’s disease brains. Here ELISA kits from Cell Signaling Technology were used to detect two phosphorylated tau biomarkers linked to Alzheimer’s disease. The study also demonstrated the utility of an ELISA kit to discriminate between the levels of the Thr181 biomarker in healthy and diseased tissue through absorbance measurements.
The benefits of the enhanced dynamic range of the CLARIOstar for chemiluminescent measurements were demonstrated in the application note Comparing site specific phospho-tau in normal and Alzheimer’s disease brains. Here ELISA kits from Cell Signaling Technology were used to detect two phosphorylated tau biomarkers linked to Alzheimer’s disease. The study also demonstrated the utility of an ELISA kit to discriminate between the levels of the Thr181 biomarker in healthy and diseased tissue through absorbance measurements.
Amplified Luminescent Proximity Homogeneous Assays like AlphaScreen® offer bead-based chemistry that can be used to study interactions between molecules in a microplate or used as immunoassays to quantify analytes. At the heart of the AlphaScreen assay is the diffusion of singlet state oxygen from donor to acceptor beads. When a biomolecular interaction takes place that brings the donor and acceptor beads into proximity, a cascade of chemical reactions ensues that amplifies the luminescent signal in the region 520-620 nm.
In the application note An AlphaScreen SureFire® Phospho-ERK1/2 assay, ERK phosphorylation is demonstrated at receptors that signal via Gi and Gq G-proteins. The AlphaScreen SureFire Phospho-ERK1/2 assay is based on a sandwich immunoassay principle. A phosphorylated cellular protein (analyte) is sandwiched between a streptavidin-coated donor bead associated with an analyte antibody and a protein A-conjugated acceptor bead associated with an anti-phosphoprotein specific antibody (Fig.9). Phosphorylation of the analyte leads to an increase in the luminescence signal. The assay was performed in a 384-well format and dose-response curves for Gi and Gq agonists were determined. 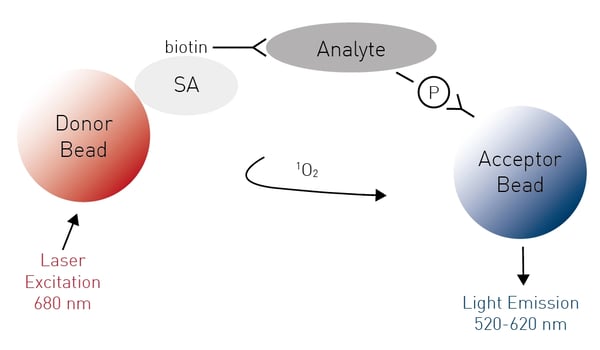
Many pitfalls can decrease the throughput, accuracy, and the overall performance of ELISAs. However, several steps can help ensure the quality of results.
The quality of starting materials is crucial for a successful ELISA. Samples must be collected properly and stored under the right conditions. Interfering substances, like antigen-binding proteins or anticoagulants, may need to be removed by centrifugation or diluted out before starting an assay. Multiple freeze-thaw cycles should be avoided by aliquoting samples before storage in a freezer.
The incubation steps where antibodies bind to antigens and substrates react with enzymes are essential for ELISAs. Optimum conditions are needed, including the right temperature, light exposure or shaking intensity. If neglected, evaporation of the well contents or a build-up of condensation on the plate sealer may occur. These discrepancies may alter the concentrations of key reactants in the wells and affect the accuracy of measurements.
Background noise can significantly interfere with ELISA measurements and non-specific binding of antibodies or antigens to microplates or deficiencies in wash steps are often to blame. Inadequate wash steps are a major culprit and it is important to wash wells thoroughly and remove the entire wash buffer after each wash step to minimize background noise.
Blocking agents for untargeted binding sites prevent detection antibodies from non-specifically binding to the surface of the microplate. Commercial blocking buffers are available for this purpose that contain unrelated large and small proteins or protein fragments. Synthetic blocking agents often provide maximal protection from non-specific interference and help eliminate background noise.
High binding polystyrene or polyvinyl chloride 96-well flat-bottomed plates are ideal for ELISAs. They help maintain consistency and minimize edge and hook effects.
Edge effects where the outer wells behave differently to internal wells may arise due to discrepancies in the manufacture of ELISA plates. Symptoms include large variations in readings for wells at the edges compared to internal wells and are best controlled by using triplicates for all samples or using evaporation lids, sealing films or tape to regulate temperature.
Hook effects occur when antigen levels are very high in the analyte. In such cases the readout is lower than expected since the level of antibody is insufficient to bind to all the antigen. Hook effects can be avoided by testing many dilutions of each sample in pilot assays before embarking on a large assay.
Assay optimization is often required when signal intensity is too high or too low. When signals are too low, which may arise due to small concentrations of antigens of interest or dilution out of interfering substances, the following may help: (i) increasing the incubation time of the enzyme-substrate reaction; (ii) increasing the amount of enzyme bound to the antibodies; (iii) increasing the quantity of antibodies to ensure that all the available substrate is bound; or (iv) increasing antibody binding times (within the defined limits of the protocol).
When the signal is higher than the detection range of the ELISA reader the following may help: (i) Sample dilution. Adding assay buffer to samples is the easiest way to decrease the concentration of analyte and to decrease the measurement intensity to a readable level. (ii) Faster addition of enzyme substrate to the wells and running the enzyme-substrate reaction in a dark incubator. High signal intensity may be due to overexposure of the enzyme substrate (typically 3,3′,5,5′-tetramethylbenzidine or TMB) to light and oxygen.
When variability among sample signal intensities is high, consider using a fluorescent or luminescent substrate and a microplate reader with a high enhanced dynamic range. A microplate reader with a high dynamic range can measure a wide range of sample signal intensities in a single detection cycle.
An ergonomic process ensures that reagents are added quickly and effectively in the right order to reaction wells. This helps ensure that samples do not dry out, which is to be avoided at all costs.
Each ELISA kit comes with a list of included materials. Most of these materials are standard, for example buffers, enzyme conjugates and wash solutions, but microplates can vary. Select the microplate that best meets the needs of the ELISA assay.
Many ELISA kits provide microplates conjugated to a specific antibody. However, coating multiuse plates with the antibodies of interest allows users to assay many different types of antigens. In this case, plates do not have to be discarded if an antibody expires. They can be recoated and used again. Multiuse plates also offer the flexibility to use multiple types of antibodies on one plate, which makes ELISAs more cost-effective.
Multimode readers offer different cost-effective solutions for ELISAs (see Table 1). Multiple enzyme-substrate pairs exist that have different reading wavelengths. Colorimetric ELISA readouts are mainly between 400 and 500 nm. If alternative substrates are used, fluorescence or luminescence signals can be employed to detect analyte concentrations. Fluorescence or luminescence methods not only exhibit a higher dynamic range, but are much more sensitive than colorimetric ELISAs, which allows for miniaturization of the assay to reduce sample costs. It is therefore cost-effective and pragmatic to use a multimode reader for ELISA measurements.
Table 1: Common enzyme-substrate combinations of ELISA assays and corresponding microplate reader requirements.
| Enzyme | Substrate | Detection Mode | Reading Wavelength (nm) |
| Horseradish peroxidase (HRP) | OPD (o-Phenylenediamine) | Absorbance | 450 (unstopped) 492 (stopped) |
| TMB (3,3′,5,5′-Tetramethylbenzidine) | Absorbance | 630 (unstopped) 450 (stopped) |
|
| ABTS ((2,2'-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]) | Absorbance | 414 | |
| Luminol | Luminescence | Detect all light | |
| Luciferin | Luminescence | Detect all light | |
| HPPA (3-p-hydroxy phenyl proprionic acid) | Fluorescence | Detect all light | |
| Alkaline Phosphatase (Alk-phos) | pNPP (p-Nitrophenyl Phosphate) | Absorbance | 405 |
| AMPPD (3-(2'-spiroadamantane)-4-methyl-4-(3'-phosphoryloxyphenyl-1, 2-dioxetane)) | Luminescence | Detect all light | |
| 4-MUP (4-methyl umbelliferyl phosphate) | Fluorescence | Excitation 360 Emission 440 |
|
| ONPG (o-Nitrophenyl-ß-D-galactopyranosidase) | Absorbance | 420 | |
| AMPGD (3-(2'-spiro ada mantane)-4-methoxy-4-(3'-ßD-galactopyranosyloxy phenyl-1,2-dioxetan) | Luminescence | Detect all light | |
| MUG ((4-methylumbelliferyl galactoside) | Fluorescence | Excitation 360 Emission 440 |
|
| Urease | Urea bromocresol | Absorbance | 590 |
The number of studies using ELISAs on BMG LABTECH microplate readers continues to grow at pace. In the research paper "Evolution of antibody immunity to SARS-CoV-2", researchers performed ELISA assays to evaluate antibody binding to the coronavirus.8 The assays were part of work designed to look at the persistence of the B cell immune response up to 6 months after prior infection with coronavirus.
In the study "An ELISA-based method for rapid genetic screens in Drosophila" published in Proceedings of the National Academy of Sciences, a CLARIOstar Plus was used to develop a genetic screening platform in Drosophila that involved a Green Fluorescent Protein-based ELISA.9 The new platform can be used to accelerate the discovery of novel genes involved in diverse biological events or to screen for regulators of subpopulations of cells, transcriptional programs, and proteins.
Future advances in immunosorbent assays for microplate readers will proceed on several fronts. The move towards homogeneous assays is likely to bring further savings on the time required to perform ELISAs. It will drive further miniaturization and help accelerate the selection of methods compatible with high-throughput screening methods.
More advanced detection technologies and innovation in specific assays should translate into further benefits for researchers. If the past is any indication, improvements to the innovation cycle should help to advance discovery and applications across the life sciences.
Whatever your requirements in ELISAs, BMG LABTECH has the microplate reader for your applications.
The PHERAstar FSX was specifically conceived for screening campaigns and is your go-to reader for high-performance high-throughput screening.
Both the VANTAstar® and CLARIOstar Plus allow for wavelength flexibility and include Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities. In addition, the Omega series, CLARIOstar Plus, and PHERAstar FSX microplate readers come with on-board injectors that can offer the very best options for detection at the time of injection.
Collectively, BMG LABTECH multi-mode readers combine high-quality measurements with miniaturised assays, short measurement times, and offer considerable savings on materials and other resources.
You can read more about ELISA microplate readers and the different offerings from BMG LABTECH in the following article ELISA Plate Readers.
Absorbance plate reader with cuvette port
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Gene reporter assays are sensitive and specific tools to study the regulation of gene expression. Learn about the different options available, their uses, and the benefits of running these types of assays on microplate readers.
Light scattering offers distinct advantages for scientists interested in immunology. Find out how the NEPHELOstar Plus is used for high-throughput immunological tests.
Kinases are a large group of enzymes which are the focus of 1/3 of all drug development efforts due to their association with multiple diseases. Read more about their role and research here.
Reactive oxygen species (ROS) may have physiological as well as pathological effects. Here we explain what ROS are and how they can be measured on microplate readers.