Introduction
Cannabinoid receptors are G protein-coupled receptors (GPCRs) that lead to diverse cellular events mediated through different cell signaling pathways. Two main types of cannabinoid receptors exist: CB1 and CB2. CB1 receptors are mainly found in the brain and central nervous system, where they play a role in regulating mood, memory, pain, appetite and motor function. CB2 receptors reside in the immune system and peripheral tissues and are mainly linked to modulating inflammation, immune responses and other functions of the body’s defence system. Cannabinoids are potential tools to combat a variety of diseases including different neurological or metabolic conditions. This interest is driving efforts to understand the pharmacology of naturally occurring and synthetic cannabinoids at the receptor level. The results to date show that when different cannabinoids bind to their receptors, they produce distinct effects depending on which signaling pathway is activated. Moreover, the signaling pathway activation depends on the agonist structure and the monomeric or heteromeric cannabinoid receptor states. Here, the PHERAstar FSX microplate reader was used to detect differential binding of natural cannabinoids to CB1 based on TR-FRET assays using terbium and the CELT-335 fluorescent ligand developed by Celtarys Research SI (Spain; fig. 1). 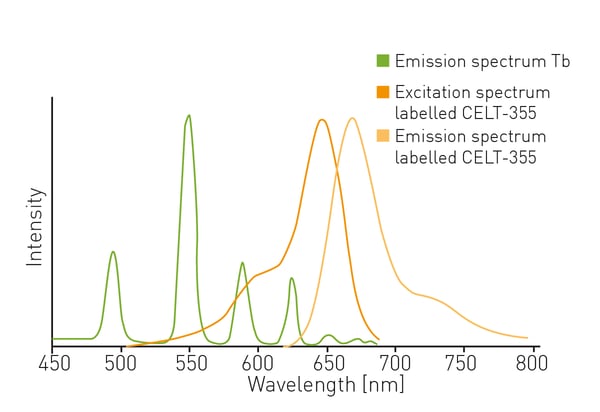 This ligand was used in assays to assess the pharmacological properties of ∆9-tetrahydrocannabinol (∆9-THC), ∆9-tetrahydrocannabinolic acid (∆9-THCA) and ∆9-tet-rahydrocannabivarin (∆9-THCV) on CB1.
This ligand was used in assays to assess the pharmacological properties of ∆9-tetrahydrocannabinol (∆9-THC), ∆9-tetrahydrocannabinolic acid (∆9-THCA) and ∆9-tet-rahydrocannabivarin (∆9-THCV) on CB1.
Assay principle
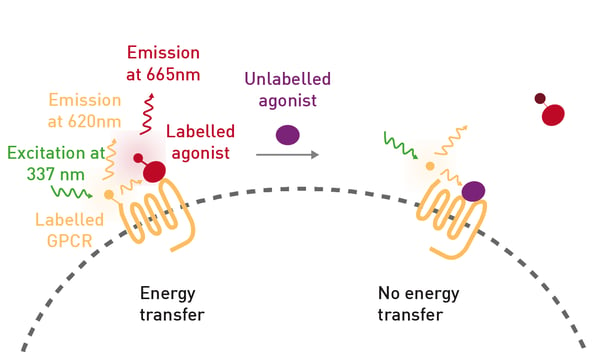
The fluorescent ligand CELT-335 is a full agonist that binds to the orthosteric site of human cannabinoid receptors and bears a highly hydrophilic fluorophore compatible with TR-FRET. The tagged agonist binds tightly to the CB1 GPCR labelled with terbium. When agonist and receptor come into proximity, energy is transferred based on a spectral overlap of terbium emission and CELT-335 excitation and light is emitted at 665 nm with terbium as the TR-FRET donor (fig. 2).- HEK-293 T cells (lot 612 968, ATCC)
- SNAP-tag® (New England Biolabs)
- Tag-lite (Cisbio-Perkin Elmer)
- CELT-335 (Celtarys Research SI)
- PHERAstar FSX microplate reader (BMG LABTECH)
Experimental procedure
HEK-293 T cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) and maintained at 37°C in a humidifi ed atmosphere of 5% CO2 as previously reported.1 Competition binding experiments were performed in HEK-293 T cells transfected with 1 μg cDNA for SNAP-CB1Ra and receptors were labelled with terbium as a TR-FRET donor. Competition binding curves were obtained by TR-FRET using 100 nM CELT-335 and 0–10 μM Δ9-THC, Δ9-THCA and Δ9-THCV.
The TR-FRET ratio was calculated based on the ratio of signal at 665 nm (acceptor) X 10,000 divided by signal at 620 nm (donor). Data were normalized to the TR-FRET ratio at the highest value (set to 100 %).
Instrument settings
|
Optic settings
|
Time-resolved fluorescence, plate mode endpoint, SDE
|
|
|
Optic Module
|
Ex: 337 |
|
|
Integration time
|
Delay: 60 µsec, Time: 400 µsec
|
|
|
General settings
|
Number of flashes | 40 |
| Settling time | 0.1 sec | |
Results & Discussion
TR-FRET assays provided sensitive and robust measurements for competition experiments looking at the binding of CELT-335 and cannabinoid substrates to living HEK-293 T cells expressing CB1. Competition binding curves were obtained using 100 nM CELT-335 and increasing concentrations of ∆9-THC, ∆9-THCA and ∆9-THCV (0 – 10 µM). Figure 3 shows that competition was similar for ∆9-THC and ∆9-THCV. The pKi values obtained for ∆9-THC and ∆9-THCV were 7.2 ± 0.6 and 7.2 ± 0.3, respectively, whereas the affinity for ∆9-THCA was considerably lower (pKi 5.8 ± 0.6).
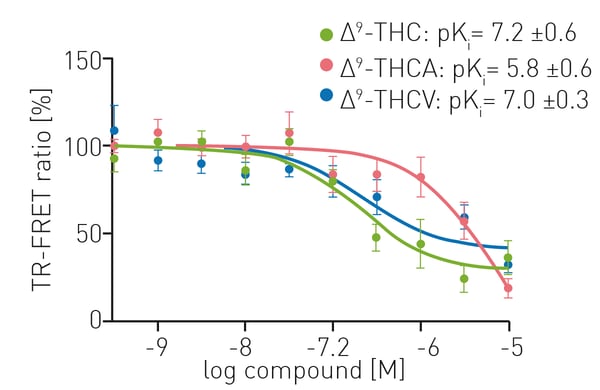 The data obtained using CELT-335 as TR-FRET acceptor are comparable to those reported in radioligand binding assays (Table 1).
The data obtained using CELT-335 as TR-FRET acceptor are comparable to those reported in radioligand binding assays (Table 1).
Table 1: Comparison of binding affi nities using radioligand binding or TR-FRET assays. Radioligand binding data are from [2,3].
| Compound |
Affinity (pKi)E
|
|
|
Radioligand binding assay
|
TR-FRET |
|
|
CB1R
|
CB1R (CELT-335) |
|
|
Δ9-THC
|
7.3-7.4
|
7.2
|
|
Δ9-THCV
|
7.12 (Ki=75.4 nM) | 7.2 (Ki=63 nM) |
|
Δ9-THCA
|
5.5 (Ki=3.1 μM) | 5.8 (Ki=1.6 μM) |
Conclusion
CELT-335 is a robust TR-FRET acceptor readily coupled with terbium as donor. CELT-335 showed high affinity for the CB1 cannabinoid receptor subtype and binding affinity values obtained for Δ9-THC, Δ9-THCA and Δ9-THCV were comparable to values reported in the literature.2,3 TR-FRET assays provide a reliable way to measure binding affi nities for cannabinoid and other receptors and the PHERAstar FSX is the ideal microplate reader to detect even the smallest changes in GPCR-ligand interactions.
References
-
Raïch I et al., Similarities and differences upon binding of naturally occurring Δ9-tetrahydrocannabinol-derivatives to cannabinoid CB1 and CB2 receptors, Pharmacological Research (2021) 174: 105970, doi: 10.1016/j.phrs.2021.105970
-
Zagzoog A et al., In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa, Scientifi c Reports (2020) 10(1):20405. doi: 10.1038/s41598-020-77175-y
-
Palomares B et al., Δ9-Tetrahydrocannabinolic acid alleviates collagen-induced arthritis: Role of PPARγ and CB1 receptors, British Journal of Pharmacology (2020) 177(17): 4034-4054. doi: 10.1111/bph.15155.