
PHERAstar FSX
Powerful and most sensitive HTS plate reader
Cannabinoids offer exciting opportunities to target diverse diseases with unmet needs. Learn how microplate readers can help improve our understanding of drug screening and drug signaling events to help advance cannabinoid research.
 Dr Barry Whyte
Dr Barry Whyte
Interest is growing in the use of cannabinoids as tools to treat a host of different diseases and conditions including cancer, neurodegenerative disorders, inflammation, autoimmune and other conditions. However, researchers need new ways to discover more cannabinoid drugs as well as develop novel approaches to improve the specificity and safety of therapeutic candidates in the developmental pipeline.
A better understanding of the receptor signaling properties of cannabinoid clinical candidates may open the door to the development of more effective and safer treatments. Advances in high-throughput drug screening and improved knowledge of drug and cell signaling pathways therefore offer opportunities to find new and improved cannabinoid therapeutics for debilitating diseases.
In this blog, we look at how researchers are using innovation in drug discovery and development to tackle some of these challenges and explore how microplate readers can be used to accelerate progress.
The diverse compounds that make up the main group of cannabinoid structures have one thing in common. The cellular events that arise from different signaling pathways originate from the binding of cannabinoids to specific cannabinoid receptors on the surface of cells. These receptors are examples of G protein-coupled receptors (GPCRs).2 It is estimated that approximately 1000 GPCRs are found in nature but cannabinoids for the most part are thought to interact with two types of these receptors: CB1 and CB2 (Fig.2).
Both CB1 and CB2 are class A, rhodopsin-like GPCRs. CB1 receptors are mainly found in the brain and central nervous system where they are crucial for regulating mood, memory, pain, appetite, and motor function.3 In contrast, CB2 receptors are located in the immune system and peripheral tissues.4 They are mainly associated with the control of inflammation, immune responses and other functions in the defense system of the body.
While researchers have developed agonists that target the different receptors, clinical drugs targeting cannabinoid receptors are still lacking due to the complexity of the drug signaling mechanisms. Further research is needed to develop more effective drug screening methods, improve understanding of the key signaling events, and facilitate the development of therapeutics with optimal safety profiles. Find out which BMG LABTECH microplate readers are ideally suited for this purpose in our dedicated GPCR brochure.
How do cannabinoids affect GPCRs? When a cannabinoid binds to a GPCR, it initiates a series of intracellular signaling events. First cannabinoids bind to the extracellular domain of the cannabinoid receptor. Upon binding of the ligand, the cannabinoid receptor undergoes a conformational change that activates the cannabinoid receptor and allows it to interact with a specific subset of heterotrimeric intracellular G-proteins.
In their activated forms, these G-proteins can inhibit or activate various effector proteins such as adenylyl cyclase, phospholipase C or ion channels depending on the specific G-protein involved. The types of events that ensue and some of the second messenger molecules involved in these processes are typical of ligands interacting with GPCRs and are described in more detail in the BMG LABTECH blog G protein-coupled receptors (GPCRs).
If one takes CB1 as an example, which is the most abundant GPCR in the brain, binding of ligand to this cannabinoid receptor primarily activates Gi proteins. This activation inhibits adenylyl cyclase which decreases second messenger cAMP levels and leads to the inhibition of gene transcription and synaptic remodeling. CB1 receptors are widely expressed on axon terminals where they can be activated by endocannabinoids. CB1 receptors can suppress neurotransmission in many neuronal circuits. These cannabinoid receptors and the endocannabinoid system can regulate a wide variety of physiological functions including learning and memory, motivation, pain and anxiety.
Researchers can probe this cascade of signaling events at many points within the cell. In this context, a wide range of interaction studies are possible through different detection technologies on BMG LABTECH microplate readers. These include not only ways to look at how proteins interact with other proteins but also research to identify small molecules that target these interactions as potential therapeutics. Interaction assays are also needed to look at binding kinetics. Experiments focused on binding kinetics are designed to investigate the interactions between two or more molecules over time and to determine binding affinities. Binding affinities can reveal how long it might take for a drug to become active or how long a drug may remain active, which is crucial information for the drug discovery process.
Microplate readers are used widely in drug screening and drug signaling studies. They allow researchers to make multiple measurements quickly and at high throughput and are therefore applicable to drug screening and drug signaling studies involving cannabinoids and cannabinoid receptors.
Access to a wide range of detection technologies is essential for cannabinoid research and drug discovery more widely. These range from absorbance and fluorescence measurements up to more advanced detection methods like Time-Resolved Fluorescence (TRF), Förster Resonance Energy Transfer (FRET), Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET), fluorescence polarization, bioluminescence resonance energy transfer (BRET) and nanoluciferase bioluminescence resonance energy transfer (NanoBRET™).
Microplate readers can also be used to routinely quantify cannabinoids which enables drug discovery research. For example, enzyme-linked immunosorbent assay kits are available for use on microplates that allow analytical quantification of natural or synthetic cannabinoids. These types of assays can be performed quickly and at high throughput.
The lack of high throughput screening techniques for the study of cannabinoid receptor ligand interaction is one of the main constraints on the discovery of more selective or biased ligands for GPCRs. Thus, researchers are interested in improved assay development that can be used at high throughput which is where microplate readers can help. Find out which BMG LABTECH microplate readers are ideally suited for this purpose in our dedicated GPCR brochure.
The therapeutic potential of a cannabinoid drug depends on its selectivity for a particular target. Researchers want to improve target selectivity by modifying drug candidates in a way that makes them more specific for the target cannabinoid receptors. Alternatively, a unique receptor activation profile can be generated that allows for the recruitment of preferred therapeutic effector proteins at the expense of those that lead to side effects. Microplate readers can therefore be used in different ways to examine drug specificity and efficacy. Here we provide a few examples of specific applications implemented for cannabinoid research.
As mentioned earlier, receptor signaling events are amenable to study on microplate readers. In the research report "Development of a membrane-bound Gi-CASE biosensor assay for profiling compounds at cannabinoid receptors" researchers describe a NanoBRET assay that is more efficient and amenable to automation compared to existing assay formats.5 The functional assay utilizes existing Gi-CASE biosensors designed for monitoring GPCR activation in intact cellular systems and the method is a good alternative to [35S]GTPγS binding assays which are costly, more laborious and rely on radioactive labelling.
This BRET-based system allows direct detection of Gαi signaling in both cells and membranes by measuring BRET between the Gα and the Gβγ subunits. Cells and membranes were treated with increasing concentrations of reference cannabinoid compounds with 10 µM furimazine added to generate the BRET signal. The signal was detected on a PHERAstar FSX and the data analyzed using the MARS software from BMG LABTECH. The membrane-based Gi-CASE NanoBRET system successfully characterized the potency and efficacy of cannabinoid receptor agonists and inverse agonists in a 384-well screening microplate format.
Further work to probe cannabinoid receptor signaling is described in the application note Differential binding of ∆9-tetrahydrocannabinol derivatives to type 1 cannabinoid receptors (CB1). Here a PHERAstar FSX® was used to make time-resolved fluorescence resonance energy transfer (TR-FRET) measurements to assess the pharmacological properties of different cannabinoids on the CB1 receptor. The studies used CELT-335, a dual fluorescent ligand that serves as a TR-FRET acceptor compatible with terbium.
CELT-335 is a full agonist that binds to the orthosteric site of human cannabinoid receptors and bears a highly hydrophilic fluorophore compatible with TR-FRET. The tagged agonist binds tightly to the CB1 GPCR labeled with terbium. When agonist and receptor come into proximity, energy is transferred based on a spectral overlap of terbium emission and CELT-335 excitation and light is emitted at 665 nm with terbium as the TR-FRET donor (Fig.3).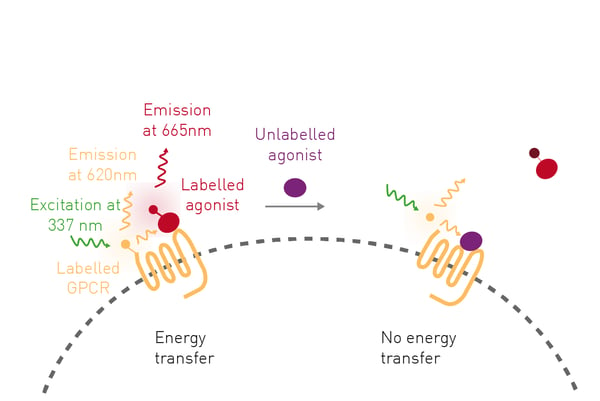
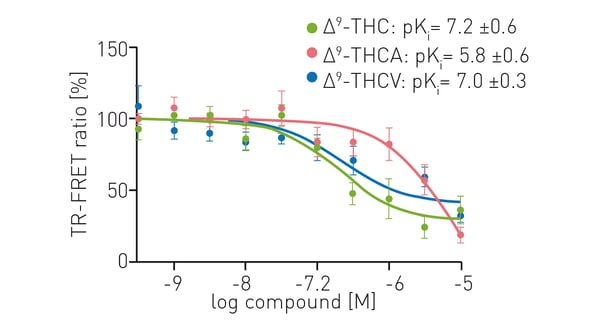
Fig.4 shows the TR-FRET competition assay between CELT-335 (TR-FRET acceptor) and ∆9-tetrahydrocannabinol (∆9-THC), ∆9-tetrahydrocannabinolic acid (∆9-THCA) and ∆9-tetrahydrocannabivarin (∆9-THCV). CELT-335 showed high affinity for the CB1 cannabinoid receptor and binding affinities for each of the cannabinoids tested were comparable to those reported in the literature. TR-FRET assays on the PHERAstar FSX therefore provided a sensitive and reliable way to measure binding affinities. The Decay Curve Monitoring (DCM) feature of the PHERAstar FSX is particularly useful for the development of TR-FRET assays for drug discovery as is the case with this approach where a novel TR-FRET acceptor was combined with the well-established donor terbium. DCM enables the collection of fluorophore decay curves for one or two wavelengths at a resolution that can be selected by the user. The dedicated photon counting detection system enables simultaneous monitoring of both donor and acceptor decay curves. Based on these curves, optimal timings for the integration start and the integration time can be selected to improve signal detection and reduce background noise.
 Applications for microplate readers are also available for events downstream of receptor binding. In this context, the application note Differential activation of G proteins by synthetic cannabinoid receptor agonists, utilizing the CAMYEL BRET biosensor describes a way to monitor second messenger cAMP levels in live cells in real time. The work focused on determining ways to establish the pathways by which some synthetic cannabinoid receptor agonists (SCRAs) might exert their toxic effects. Safety is a recurring theme for any drug discovery effort. Synthetic cannabinoids represent one of the most rapidly growing classes of new psychoactive substances associated with drug misuse. By understanding the precise mechanisms for these toxic effects researchers hope to come up with ways to mitigate these undesirable features.
Applications for microplate readers are also available for events downstream of receptor binding. In this context, the application note Differential activation of G proteins by synthetic cannabinoid receptor agonists, utilizing the CAMYEL BRET biosensor describes a way to monitor second messenger cAMP levels in live cells in real time. The work focused on determining ways to establish the pathways by which some synthetic cannabinoid receptor agonists (SCRAs) might exert their toxic effects. Safety is a recurring theme for any drug discovery effort. Synthetic cannabinoids represent one of the most rapidly growing classes of new psychoactive substances associated with drug misuse. By understanding the precise mechanisms for these toxic effects researchers hope to come up with ways to mitigate these undesirable features.
The measurement of cAMP levels was used to follow the activities of different cannabinoids as they interacted with their GPCRs. In this study, researchers were interested in testing 16 different cannabinoids to see if they signaled via the Gi or Gs pathways. If signaling proceeds via the Gi pathway a decrease in cAMP levels is observed. If the Gs pathway is involved, cAMP levels increase.
A CAMYEL BRET biosensor was used for BRET measurements that allowed changes in the levels of cAMP to be measured. This biosensor is composed of an exchange protein activated by cAMP (EPAC) bound to a luciferase (RLuc) and yellow fluorescent protein (YFP) (Fig.5).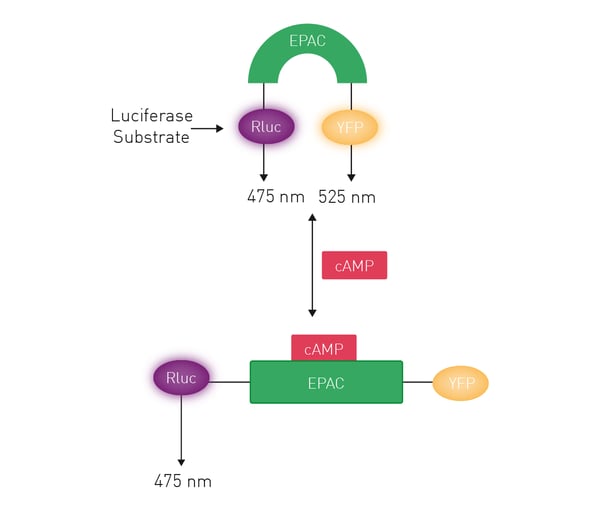
In the absence of cAMP, RLuc and YFP are in proximity due to the conformation of EPAC. A BRET signal arises when coelenterazine, the substrate of RLuc, is added. As cAMP is formed in the cell, it binds to EPAC leading to a conformational change that decreases the BRET signal as the levels of cAMP increase. The real-time measurements of the stimulation of cAMP levels by 10 µM tetrahydrocannabinol (THC), 2-arachidonylglycerol and AB-FUBINACA when added to HEK-CB1 cells are shown in Fig.6. Kinetic BRET assays were successfully performed using the PHERAstar FSX which offers considerable time savings thanks to its Simultaneous Dual Emission (SDE) feature. SDE allows two wavelengths to be read simultaneously which makes it ideal for high-throughput applications in all reading modes.
As an alternative to the use of customized filters, the CLARIOstar® Plus and VANTAstar™ also offer very high light transmission and sensitivity through their Linear Variable Filter (LVF) Monochromators™ which make them excellent choices for luminescence and BRET measurements. The flexibility of this LVF monochromator allows bandwidths up to 100 nm to be selected, which further increases assay sensitivity. An example where the CLARIOstar is used for nanoBRET detection involving filters or the LVF monochromator is described in the application note CRISPR/Cas9 genome-edited cells express nanoBRET-donor that monitors protein interaction and trafficking. Here CRISPR/Cas9 genome editing was used to enable endogenous expression and regulation of BRET donor-labelled proteins. Two proteins fused to different acceptor fluorophores were used to monitor receptor trafficking.
Safety was also the focus in the research report "Subtle structural modification of a synthetic cannabinoid receptor agonist drastically increases its efficacy at the CB1 receptor" where researchers looked at the impact of structural changes to synthetic cannabinoids on their efficacy and potency at CB1 receptors.6 Synthetic cannabinoid receptor agonists offer opportunities for the development of new therapeutics. However, the emergence of synthetic cannabinoid receptor agonists as illicit psychoactive substances also poses risks to public health and has led to some deaths. Small changes to the structures of phytocannabinoids such as ∆9-tetrahydrocannabinol can lead to dramatic differences in drug signaling levels that can be toxic.
In this study, researchers investigated the structure-activity relationships of aminoalkylindole SCRAs at the CB1 receptor. Specifically, the study focused on changes to the ligand head region of the SCRAs. A PHERAstar FSX was used to perform BRET assays designed to detect ligand-induced receptor signaling protein coupling events. Both G-protein engagement and beta-arrestin recruitment were monitored. For kinetic experiments, donor luminescence and acceptor fluorescence were quantified with BRET signal measurements taken at various time points in the window from 2 to 46 minutes. The PHERAstar FSX provided a robust platform with the high sensitivity needed for the BRET measurements.
The study demonstrated that minor structural changes in the head moiety of SCRAs can cause major changes in efficacy (Fig.7). A substantial increase in the efficacy and potency of a 5F-pentylindole SCRA was evident by the addition of a single methyl group to the head moiety of the SCRA. An increase in efficacy and potency may also increase the risk of adverse and toxic affects. The results highlight the need for close monitoring of the structural modifications of newly emerging SCRAs and their potential for toxic drug responses in humans.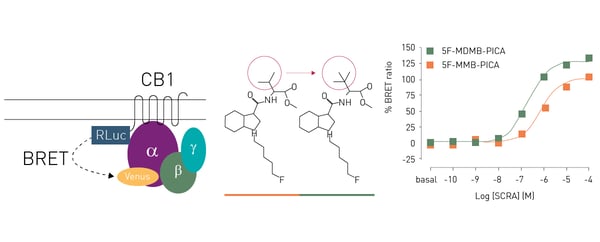 You can learn more about how microplates are being used for cannabinoid research in the interview The benefits of studying opioid, cannabinoid, terpenoid, and 5HT receptor agonists. Here Dr. Marina Junqueira Santiago describes some of the research into cannabinoids underway in her laboratory and some of the potential benefits of this type of work for drug discovery and development.
You can learn more about how microplates are being used for cannabinoid research in the interview The benefits of studying opioid, cannabinoid, terpenoid, and 5HT receptor agonists. Here Dr. Marina Junqueira Santiago describes some of the research into cannabinoids underway in her laboratory and some of the potential benefits of this type of work for drug discovery and development.
In this type of work, the PHERAstar FSX is frequently used to look at binding events, for example when a ligand binds to a receptor, or to measure second messengers involved in the signaling cascade. By offering access to a wide range of the detection technologies mentioned earlier in this blog, the PHERAstar FSX can be readily used to test new assays and look at the different ways that signaling pathways are activated. The PHERAstar FSX offers SDE detection and provides considerable flexibility when new assays are needed through the ready availability of different optic modules. New assays and developments will help to optimize productivity, improve safety as well as enhance cannabinoid specificity and efficacy.
What is the preferred BMG LABTECH microplate reader for specific needs and applications related to cannabinoid research? BMG LABTECH offers a range of detection devices for sensitive fluorescence and luminescence measurements.
The PHERAstar FSX was specifically conceived for screening campaigns and is an ideal reader for high-performance, high-throughput investigations. The PHERAstar FSX combines high sensitivity and speed and can read two wavelengths simultaneously which makes it ideal for high-throughput applications in all reading modes. In addition to cutting read times in half, Simultaneous Dual Emission helps reduce data variability caused by fluctuations in pH, temperature or evaporation. Further, the PHERAstar FSX comes equipped with dedicated light sources including an in-built laser for TRF/TRF-FRET and one for AlphaScreen® applications.
Both the VANTAstar® and CLARIOstar® Plus allow for wavelength scanning and include Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options. In addition, they can be equipped with the Atmospheric Control Unit for live cell-based assays.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities and fast detection times up to 0.01 s are readily available. In addition, the Omega series, CLARIOstar Plus, and PHERAstar FSX microplate readers come with on-board injectors that can offer the very best options for detection at the time of injection. The VANTAstar can be used with an injector module combined with a heater and stirrer providing further application opportunities.
Collectively, these multi-mode readers combine high performance with miniaturized assays, short measurement times, and offer considerable savings on materials and other resources.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Degrons are specific sequences of amino acids or structural motifs within a protein that are important for targeted protein degradation. Find out how microplate readers can advance research into natural and engineered degrons.
Gene reporter assays are sensitive and specific tools to study the regulation of gene expression. Learn about the different options available, their uses, and the benefits of running these types of assays on microplate readers.
Innovation for targeted protein degradation and next-generation degraders is gathering pace. This blog introduces some of the different approaches that act via the lysosome or proteasome.
The choice of assay for targeted protein degradation studies is crucial. But what is the preferred assay and detection technology for your specific research needs and microplate reader?
Molecular glues are small molecules that help target unwanted proteins for destruction by the ubiquitin-proteasome system. Find out how microplate readers can advance molecular glue research.