Introduction
Viruses are obligate intracellular pathogens that can infect all the living organisms. They not only assume an outmost medical relevance due to their connection with several human diseases, but they also have a major economic impact given the costs required for medical treatments and for protecting cattle and crops. Viruses encode a limited number of proteins, and they thus interact and hijack host cellular machineries to promote their replication and spread. Therefore, understanding host-virus interactions become crucial to uncover the molecular mechanisms underpinning infection, and for the development of new treatments. With the emergence of ‘omics’ in the last decade, known host-virus interactions have been massively expanded, calling for novel approaches to test the importance of cellular proteins in virus infection in a systematic way. Moreover, the discovery of antivirals often relies on the screen of large libraries of compounds, which also poses a technical challenge. Hence, to test the importance of cellular proteins in virus infection and to discover new antivirals, robust and efficient high-throughput methods are required to measure viral fitness without using expensive reagents such as luciferin.
Here we describe a method to analyse viral gene expression in near real-time using fluorescence as a proxy for infection. This assay can discover not only if a given condition inhibits or enhances virus infection, but also provides insights into its effects on the kinetics of infection.
Assay Principle
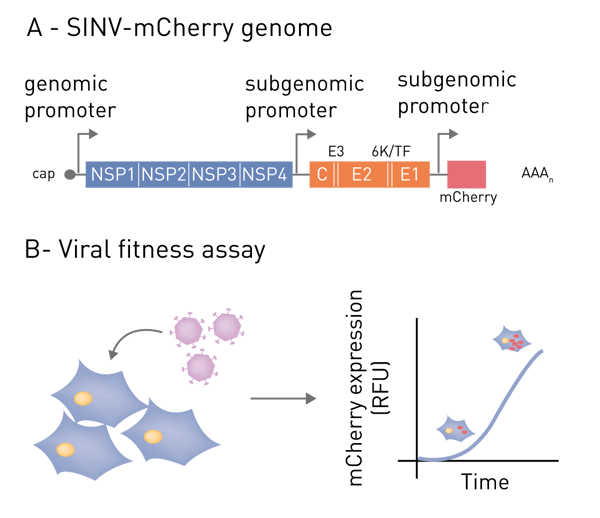 This application note illustrates a fluorescent-based method for monitoring viral gene expression using a BMG LABTECH CLARIOstar Plus microplate reader with Atmospheric Control Unit (ACU). As an illustrative example, we employ Sindbis virus (SINV, Alphavirus genus) with engineered genome containing the coding sequence for mCherry fluorescent protein. Expression of mCherry can be used as a proxy for viral gene expression and can be measured at regular intervals throughout the infection. Although exemplified with SINV, this approach has been applied successfully to a broad range of viruses including the human immunodeficiency virus (HIV).
This application note illustrates a fluorescent-based method for monitoring viral gene expression using a BMG LABTECH CLARIOstar Plus microplate reader with Atmospheric Control Unit (ACU). As an illustrative example, we employ Sindbis virus (SINV, Alphavirus genus) with engineered genome containing the coding sequence for mCherry fluorescent protein. Expression of mCherry can be used as a proxy for viral gene expression and can be measured at regular intervals throughout the infection. Although exemplified with SINV, this approach has been applied successfully to a broad range of viruses including the human immunodeficiency virus (HIV).
Materials & Methods
- 96-well microplate, flat µclear bottom (Greiner Bio-One). This protocol is, also compatible with 384-well microplates.
- Colourless DMEM medium
- SINV-mCherry virus stock (~5x108 pfu/ml)
- BMG LABTECH CLARIOstar Plus microplate reader with ACU
Experimental Procedure
HEK293 cells were seeded at a density of 5x104 cells in a 96-wells microplate with flat µclear bottom. Infection was performed using SINV-mCherry at 0.1 multiplicity of infection (MOI), and cells were incubated at 37° C with 5% CO2 in the CLARIOstar Plus plate reader for 24 hours. Incubation time should be adapted to the lifecycle duration of each virus (e.g. 72 hours for HIV). The viral genome expression was monitored by the detection of the red fluorescent signal derived from mCherry (excitation: 570 nm, and emission: 620nm), which was measured every 15 minutes. The addition of drugs or other reagents (e.g. interferon) can be performed before or after starting the programme in the plate reader.
Instrument settings
| Optical settings | Filters |
Excitation 570-15
Dichroic 593.8 Emission 620-20 |
| Focal height | 4.4 mm | |
| Gain | 2900 | |
| General settings | Number of flashes | 8 |
| Settling time | 0 s | |
| Scan settings | Orbital averaging | |
| Scan diameter | 4 mm | |
| Kinetic settings | Number of cycles | 92 |
| Cycle time | 900 s | |
| Incubation 37° C, 5% CO2 | ||
Results & Discussion
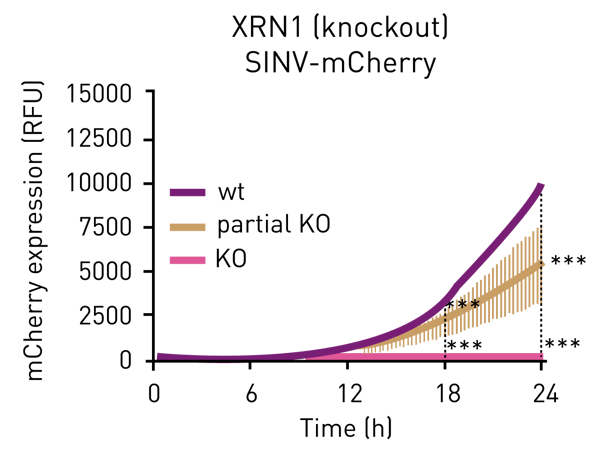
To assess viral genomic replication we infected WT (wild type), XRN1+/- (partial knockout), and XRN1-/- (complete knockout) cells with SINV-mCherry, and the red fluorescence protein was monitored every 15 minutes for 24 hours using the CLARIOstar Plus microplate reader. The detection of fluorescent signal every 15 minutes for 24 hours post infection (hpi) was enough to cover both, the early and late phases of SINV infection. In HEK293 WT cells, SINV gene expression was detected at around 8 hpi with a subsequent constant increase (figure 2). In cells completely lacking XRN1, viral gene expression drops to the background level. These results reflect that XRN1 is a critical host factor for SINV infection and the lack of it causes cells to become refractory to infection1 (figure 2).
The high sensitivity of the assay allows for the profiling of intermediate phenotypes in a very reproducible manner. For example, when XRN1 is partially depleted, SINV infection is delayed but not fully supressed1 (figure 2).
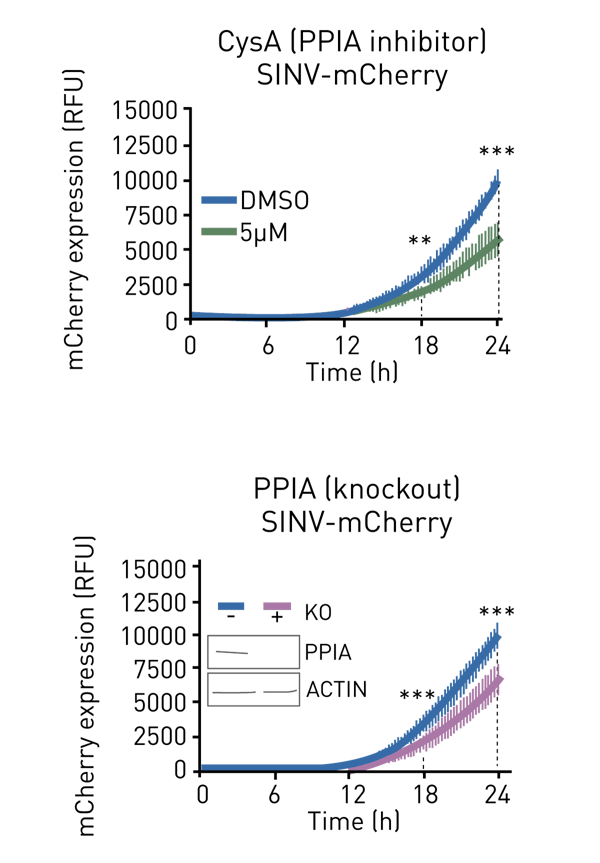
In addition, this assay allows the testing for drug effects on viral fitness. For example, figure 3 shows that cyclosporin A, a known inhibitor of cyclophilin A (PPIA), moderately delays SINV gene expression to a comparable level to that of a PPIA knockout (figure 3, bottom panel). This example goes to highlights the potential of this application for screening the effects of proteins and drugs on viral gene expression.
Conclusion
The viral fitness assay can be successfully performed using the CLARIOstar and CLARIOstar Plus fluorescence microplate reader with ACU permitting the study of viral gene expression in multi-well plates in real-time for up to 72 hours post infection. This method allows for the simultaneous screening of a broad range of experimental conditions, such as drug libraries and knock-out cell lines in virus infection.
References
- Garcia-Moreno, M. et al. System-wide Profi ling of RNA-Binding Proteins Uncovers Key Regulators of Virus Infection. Mol Cell, doi:10.1016/j.molcel.2019.01.017 (2019).