
PHERAstar FSX
Powerful and most sensitive HTS plate reader


Microplates come in different colours, materials, well densities and geometries, explore which format is best suited for your applications.
Microplates are laboratory equipment that consist of a plate with multiple cavities (wells) used as small sample tubes. They are also referred to as microtiter plates or multi-wells and typically come in 6-, 12-, 24-, 48-, 96-, 384- or 1536-well formats with the 96-well layout being the most commonly used. Depending on the well size, they can hold microliter to millilitre volumes of sample.
Microplates facilitate the preparation, handling, examination, processing, and management of many samples. Today, they are a standard in life science and pharmaceutical research as well as in clinical diagnostics and analytical research. Enzyme-linked immunosorbent assays (ELISA) are one of the most commonly used applications. However, uses are diverse and widespread in the life sciences including sample storage, filtration, separation and mixing, as well as sample detection and quantification.

Microplates were created in 1951 by Hungarian microbiologist Dr. Gyula Takátsy (fig.1), as an influenza epidemic led to a shortage of laboratory equipment. Takátsy needed to find inexpensive solutions to proceed with his clinical tests. He noticed that testing was too expensive in large batches and that a significant increase in throughput could be achieved by placing multiple samples next to each other. Accordingly, he created the first microplate by putting 8 rows of 12 wells (8*12 = 96) together by hand and linked them with a wire loop. This solution enabled him to save time when pipetting.
In the 1960s, his invention gradually gained in popularity and was adopted as standard equipment in biomedical testing by laboratories worldwide.
Widespread usage was further facilitated in the late 1980s as a moulded version was introduced. This decreased the price per unit and increased production capacity. In the following years, microplates were further improved and multiple variants were developed to fit the different needs of the scientific community.
In the 1990s, the Society for Biomolecular Screening (SBS) started an effort to standardise features and shapes. Features mainly included the number, shape and dimension of wells, as well as properties such as general dimensions and material. In 2003, the American National Standards Institute (ANSI) proposed and published a set of standardised features, which included well diameter, depth and spacing, as well as general dimensions (i.e., 127.76 mm × 85.48 mm).1
Standardisation led to wider use, particularly benefitting lab automation as well as the production of lab instrumentation. Consequently, the microplate further grew in popularity as an effective support for drug discovery and life science research. It was estimated that around 125,000,000 units were used in 2000 alone.2
In 2010, the SBS and the Association for Laboratory Automation (ALA) merged to form the Society for Laboratory Automation and Screening (SLAS). Since then, microplate standards are referred to as ANSI/SLAS.
Besides the number of wells, microplates come in different materials, colours, and include different well shapes and volume capacities. With plenty of options, it is sometimes hard to pick the optimal one for a specific assay. Here, I will try to summarise the main features while keeping the main focus on spectrophotometric applications.
Although there are small differences in size and well position for the different types of microplates from different manufacturers, all microtiter plates come with the same footprint dimensions. Wells are usually arranged in a rectangular matrix and typically come in 6-, 12-, 24-, 48-, 96-, 384- and 1536-well formats (fig. 2). A 3456-well layout is also available but is not often used.
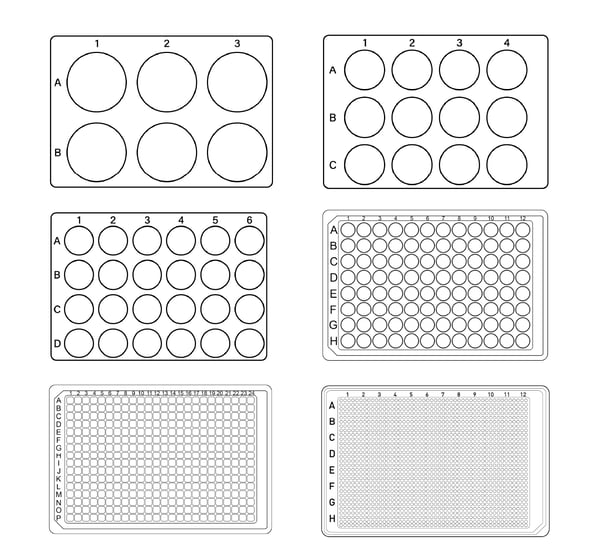 The fill volume for each well depends on the layout. The most used layout is the 96-well format (up to 300 µL/well). Higher density layouts are usually employed for miniaturization purposes. Besides bearing more samples, 384- (up to 100 µL/well), 1536- (up to 15 µL/well), and even 3456-well formats (up to 5 µL/well) reduce reagent volumes and consequently costs (Table 1).
The fill volume for each well depends on the layout. The most used layout is the 96-well format (up to 300 µL/well). Higher density layouts are usually employed for miniaturization purposes. Besides bearing more samples, 384- (up to 100 µL/well), 1536- (up to 15 µL/well), and even 3456-well formats (up to 5 µL/well) reduce reagent volumes and consequently costs (Table 1).
As a general rule, one-third of the maximum fill volume is the lowest volume recommended for a well to have an efficient and realistic measurement. Hence, you should not go below 100 µL for a standard 96-well microtiter plate.
Half-area 96-well microplates (up to 170 µL/well) are intermediate solutions. These essentially have the same well size as the 384-well layout while keeping the outer dimensions and well positions of a 96-well. Accordingly, wells are smaller and allow a reduction of up to 50% in sample volume. This format is typically used as an alternative to the 384-well when low volumes are required but automation and throughput are not necessary.
A similar concept is applied to 384-well layouts in low- or small-volume format (sometimes also referred to as “high base”). These plates have the same well size as a 1536-well layout with the outer dimensions and well positions of a 384-well plate. Likewise, sample volumes can be reduced by more than 50% when compared to a standard 384-well. In addition to volume, well shape is also affected: while regular 384-well layouts have square-shaped wells, low-volume have round ones.
Table 1: Comparison of the different fill volume capacities of different layouts.
| Well number | Recommended volume |
| 6 | 2-5 mL |
| 12 | 2-4 mL |
| 24 | 0,5-3 mL |
| 48 | 0.5-1.5 mL |
| 96 | 100-300 µL |
| 96 half area | 50-170 µL |
| 384 | 30-100 µL |
| 384 low/small volume | 5-25 µL |
| 1536 | 5-25 µL |
| 3456 | 1-5 µL |
In general, higher density formats (i.e., 1536- and 3456-well) cannot be pipetted by hand and a pipetting machine is needed. Manual pipetting is still possible in 384 wells but tedious and usually not recommended. Consequently, one must weigh whether the higher handling costs associated with working with 1536- or 3456-well microplates are counterbalanced by the cost savings achieved by the miniaturization. This is typically the case for high-throughput screening facilities but not for most life science labs.
Plates for ELISA typically come with 96 wells and consist of twelve separate eight-well strips (fig. 3). This architecture simplifies partial use and handling.
Microtiter plates can be manufactured from different materials. The most commonly used are the polymers polycarbonate, polystyrene and cycloolefin. The choice of polymer affects light transmission, autofluorescence, water absorption and gas exchange, and consequently influences use, applications, and assay quality.
Typically, the most used material for plastic labware is polystyrene. Its transparency makes it ideal for optical detection, absorbance assays (such as ELISA) and microscopy with moderate modification. However, polystyrene does not transmit UV light (< 320 nm) and is hence inappropriate for RNA and DNA quantification. For this purpose, cyclicolefin copolymer (COC) comes with improved ultraviolet light transmission in the range 200 – 400 nm and with low autofluorescence (fig. 4).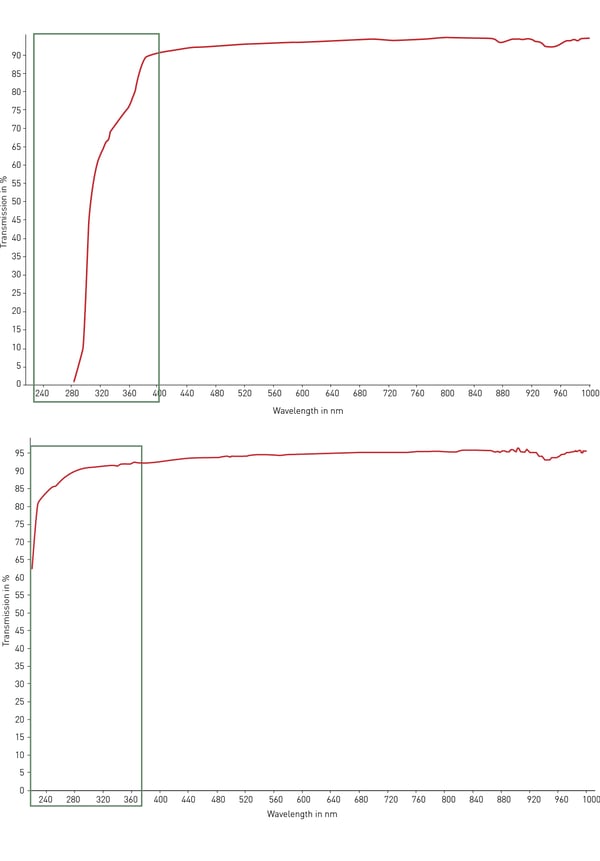 For optical and microscopy applications as well as high-content screening, glass and quartz are the best possible materials for transparency, autofluorescence and light transmission. However, these materials are very expensive, fragile and are usually not disposable.
For optical and microscopy applications as well as high-content screening, glass and quartz are the best possible materials for transparency, autofluorescence and light transmission. However, these materials are very expensive, fragile and are usually not disposable.
Besides optical properties, water absorption and gas exchange play an important role for cell-based applications. In addition, the mechanical properties affect handling, especially by automated systems. Thermal properties can play a role in high-temperature applications (e.g., PCR plates) or when sealers are need.
Polycarbonate and polypropylene are mainly used for PCR plates (fig. 5) and for samples subject to wide changes in temperature (e.g., −80°C storage). Besides temperature stability, resistance to numerous solvents and biological inertness are also required for storage purposes.
Microplates typically come in four colours: clear, black, white, and grey (fig. 6). Depending on the used detection mode, colour may affect signal-to-background ratios and well-to-well cross-talk when very bright and dim samples are adjacent. Accordingly, a suboptimal colour choice can have a negative impact on your data.
 Clear (including UV-transparent) microplates are required for absorbance assays as light has to pass through the sample in this detection mode.
Clear (including UV-transparent) microplates are required for absorbance assays as light has to pass through the sample in this detection mode.
Black microplates (which are darkened by the presence of carbon) partially quench the signal of the sample. This happens because the black colour partially absorbs the light signal coming from the sample. Consequently, these plates are well suited for fluorescence intensity including FRET, and for fluorescence polarization assays. These are usually detection modes with a strong signal yield and the use of the black colour helps to reduce background, auto-fluorescence and well-to-well cross-talk, providing better signal-to-blank ratios.
Black microplates are generally not recommended for luminescence, time-resolved fluorescence (TRF) and TR-FRET assays because these detection modes usually have a comparably low signal yield that would be further quenched by the black colour. White plates, which include titanium dioxide, are recommended for these assays. The white colour of the well partially reflects the sample signal helping to maximise it. The drawback is that white microplates also increase the blank signal. However, this is usually quite low in luminescent assays. In TRF, the delayed measurement window eliminates the influence of the background.
An intermediate solution between black and white are grey microplates. These are specifically recommended for AlphaScreen® and AlphaLISA® as they reduce cross-talk and background while still providing good signals.
If you would like more details about the influence of plate colour on the measurement results in different detection modes and would like to see data proving the effects, please visit HowTo Note 6: How to choose the best microplate colour for my assay
Wells can be either circular or square with the latter having either straight or rounded corners. Square wells contain a larger sample volume and increase the light transmission area compared to round wells. Round wells have a smaller total area and are better suited for shaking. Moreover, as round wells normally do not share a common wall with adjacent wells, they are less affected than square ones by signal cross-talk through the plastic wall of the well.
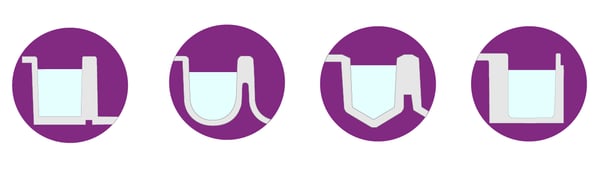
Well bottoms also come in different shapes: F-, V-, U- and C-bottom (fig. 7). F-bottom (flat) wells provide the best light transmission, are ideal for adherent cell cultures and are suited for bottom-reading assays with plate readers or microscopes.
V-bottom (conical) wells enable maximal volume retrieval of small and precious samples because of their shape. However, the conical shape is disadvantageous for spectrophotometric or imaging applications. Accordingly, V-shaped wells are mainly used for sample storage and precise pipetting.
A rounded well bottom (U-bottom) facilitates mixing, washing, and coating. U-bottom wells enable easy and residue-free pipetting and are typically used for cells in suspension and spheroids.
Curved bottom (C-bottom) wells are a compromise between F-bottom and U-bottom wells. The flat bottom makes them suitable for optical measurements, while the rounded edges facilitate mixing and washing.
The surface properties of the well play an important role. Here, the sample and the well material interact with each other. Typically, three main types of microplates are available: non-binding or low-binding, medium-binding, and high-binding. Binding is affected by the physical properties of the material and can be modified by applying different coatings.
For biochemical spectroscopy and high-throughput screening applications, polystyrene with no coating is typically the material of choice and categorised as medium-binding.
Non-binding plates prevent binding of nucleic acids, proteins and peptides to the surface of the well. Here, a specific coating decreases assay background and improves signal-to-noise.
High-binding microplates are typically used for assays that require the immobilisation of molecules to the surface of the well. One of the best-known uses are ELISA assays. Here, specific coatings and treatments introduce a defined number of hydrophilic groups to facilitate passive adsorption of biomolecules (e.g., antibodies). Medium- and high-binding plates typically differ in the number of available polar groups.
One example of how plate coating can affect biological data was shown by means of a proteasome activity test run with non-binding, medium-binding and high-binding plates. In this study, the researchers obtained different results and showed that the performance of the proteasome assay was affected by the properties of the microplates. Proteasome activity was determined with a fluorescent proteasome substrate, 7-amino-4-methylcoumarin (AMC), a widely used label for this purpose. As the excitation and emission spectra of bound and unbound AMC are different, this allowed the free AMC released by proteolytic cleavage to be easily detected with a fluorescence plate reader. However, standard curves of free AMC showed significant differences among the three plate types. As expected, the high-binding plate gave the lowest signal. Different microplates should therefore be evaluated to determine the best one for the assay as well as the role of any possible plate-specific effects.4
Different plate-coating may also have a substantial influence on the formation of menisci at the liquid surface which may substantially impact absorbance-based measurements due to a changed path length as explained in the HowTo Note: How to deal with path length and meniscus in microplates.
Regular plates cannot be used for cell culture and cell-based assays as they are typically non-sterile. In addition to sterility, specific hydrophilic surface treatments are required to facilitate cell attachment to the bottom of the well. Such hydrophilic treatments are usually referred to as “tissue culture-treated” or TC-treated. There are also coatings based on extracellular matrix proteins like collagen, fibronectin and laminin, as well as synthetic coatings like with poly-lysines. A cell-repellent surface is typically required for suspension cells, organoids and spheroids.
Since cellular assays are sometimes evaluated over several hours or days, evaporation from the cell-containing wells can potentially influence the results and ultimately lead to decreased cell viability. Some manufacturers offer plates including an outer moat that can be filled with liquid. This helps to insulate the wells, thereby preventing evaporation especially from edge wells that pose a risk of evaporation.
When running cell-based assays, detecting from the bottom of the well is usually recommended for several reasons. In regular white or black plates, reading from the bottom of the well is not possible and clear bottoms must be used. The walls of the wells can be white or black depending on the assay. The clear bottom can be made of different materials — from plastic (polystyrene or cyclicolefin copolymer), up to glass or quartz. Although detection is significantly better with glass and even more so with quartz compared to plastic, these are also significantly more expensive. Moreover, glass and quartz plates are not disposable and have to be cleaned and autoclaved to sterilise after every use. Hence, one has to decide whether the better performance that comes at a higher cost and with more maintenance, justifies the use of such a plate compared to a cheaper, easy-to-use disposable microplate with a clear plastic bottom.
Microplate readers detect and quantify light signals produced by liquid samples in microplate-based assays. These laboratory instruments are used in the life sciences and pharmaceutical industries to quantify chemical, biological or physical reactions. In addition to biological, cellular, biochemical, pharmaceutical and drug discovery applications, microplate readers are also used in environmental research, and in the food or cosmetics industry.
Microplate readers either come as single- or as multi-mode instruments capable of quantifying samples in different modes such as absorbance, fluorescence intensity, luminescence, time-resolved fluorescence, TR-FRET, fluorescence polarization and AlphaScreen. Depending on their method of wavelength selection, plate readers can be either monochromator- or filter-based.
Their use typically improves efficiency in laboratory practices and helps to save reagent costs and reduce operational time. For an overview about how microplate readers are used today, please have a look at our application note database.
Despite ANSI/SLAS standards, plates of different manufacturers come with slight variations in dimensions and well positions. This can influence the measured data. Even a small offset in the depth or width of the well position over a line with 24 or 48 wells (for a 384- and 1536-well plate, respectively) may result in measurements no longer being made in the centre of the well but in the worst case on the plastic wall between two samples.
Accordingly, BMG LABTECH readers come with a microplate selection database that includes more than 50 different plate layouts from different manufacturers.
In addition to microplate readers, there are other different instruments that handle or manage microplates. These instruments are specifically used in lab automation. A few examples include liquid handlers and washers used to dispense or remove liquids from and to different plates, robotic arms and conveyor belts to transport plates between instruments, plate stackers to store them for short times and plate hotels or incubators for longer-term storage. In addition, plate sealers and de-sealers apply and remove sealing foils. An example of automated handling can be seen in the following video:

Collectively, BMG LABTECH multi-mode readers combine performance with miniaturized assays, compatibility with automated handling options, short measurement times, and result in savings on materials and other resources.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Flexible microplate reader with simplified workflows
Upgradeable single and multi-mode microplate reader series