
PHERAstar FSX
Powerful and most sensitive HTS plate reader
Neurodegenerative disease ultimately leads to neuronal cell death as neuronal functions deteriorate. Find out how microplate readers can be used to study neuronal cell death and its contributions to neurodegenerative disease.
 Dr Barry Whyte
Dr Barry Whyte
Cells can die in many ways and neurons are no exception. Some of the main routes to destruction for neuronal cells include apoptosis, necrosis and autophagy.1 Each has its own distinctive features, but they also share some common ground at the molecular level. Significantly, neuronal cell death is a known contributor to neurodegenerative diseases like Parkinson’s and Alzheimer’s disease and also impacts amyotrophic lateral sclerosis (ALS).2 However, the precise interplay of the different cell death mechanisms and processes that contribute to neurodegenerative disease, including oxidative stress, mitochondrial dysfunction , protein aggregation and inflammation, remain to be established.
In this blog, we look at the relationship between neuronal cell death and neurodegenerative disease. We also discuss how microplate readers can be used in neuroscience research to study the molecular processes that contribute to neuronal cell death.
Neurons may die from programmed cell death (apoptosis), receive a barrage of external factors that lead to their demise (necrosis), or have their constituents recycled to a point that fatally compromises the cell (autophagy) (Fig. 1). Here we look briefly at these three types of cell death.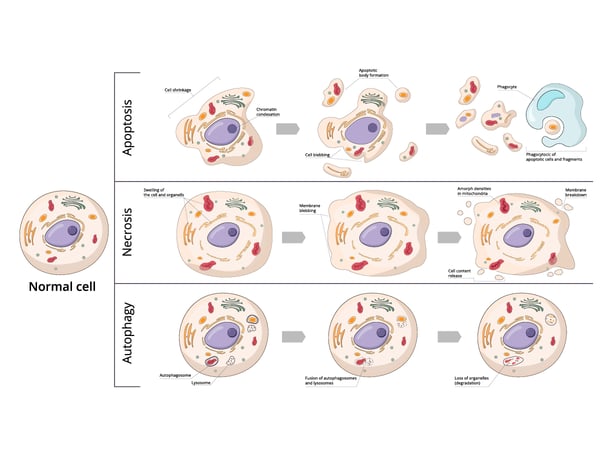
Apoptosis is a form of programmed cell death. In apoptosis, cells activate a series of intracellular signals that lead to their own demise. In the context of neuronal cells, apoptosis plays a significant role in the regulation of neuronal populations not just in development but also in the elimination of injured or unnecessary neurons. Unlike necrosis, apoptosis is a natural built-in process of the cell and is not caused by external factors.
Necrosis typically occurs in response to acute cellular injury such as physical trauma, infection, or lack of oxygen. Unlike apoptosis, necrosis is an unplanned process. It often involves cellular swelling, rupture of the plasma membrane, and release of cellular contents which can trigger an inflammatory response. Necrosis can occur in neuronal cells under certain conditions such as stroke or traumatic brain injury where the disruption of the blood supply or physical trauma leads to cellular damage and death.
Autophagy is a cellular recycling process that involves the degradation and repurposing of damaged or dysfunctional cellular components. Autophagy can protect the cell from harmful proteins or damaged organelles. At the same time, excessive autophagy can lead to cell death. In neuronal cells, autophagy is essential for maintaining homeostasis and cell function.
While apoptosis, necrosis, and autophagy each have their distinct features, each of these types of cell death is not mutually exclusive and can interact with each other in many ways at the molecular level.
Neuronal cell death is also closely linked to oxidative stress, mitochondrial dysfunction, protein aggregation and inflammation. These processes form a vicious cycle, each one influencing the other. For example, oxidative stress can lead to mitochondrial dysfunction which in turn generates more reactive oxygen species and exacerbates oxidative stress. The interplay between these processes contributes to the onset of neurodegenerative disease and eventual neuronal cell death.
Neurodegenerative diseases like Alzheimer’s, Parkinson’s disease, and ALS are characterized by the progressive loss of neurons in specific regions of the brain or spinal cord. The involvement of specific cell death mechanisms plays a role in this destruction. Functional impairments arise from the loss of critical neuronal cell populations and their connections in specific areas of the brain. Neuronal damage is typically a progressive process. As more neurons die, the disease gradually worsens over time. Many of the diseases are associated with the accumulation of toxic protein aggregates like tau, amyloid-beta and alpha-synuclein.
Neuronal cell death can lead to the loss of both pre-synaptic and post-synaptic neurons which results in a reduction in synaptic connections. Synapses are crucial points of communication between neurons where electrical and chemical signals are transmitted. Therefore, synaptic loss can impair the overall connectivity and integration of neuronal circuits.
The death of specific neuronal populations disrupts the neuronal circuits in the brain and alters the balance of excitation and inhibition of neurons. This disruption can lead to the loss of information processing, impaired signal transmission, and reduced functional efficiency within affected brain regions. The consequences of synaptic loss and network disruption manifest as functional impairments in the cognitive, sensory and motor domains. In Alzheimer’s, synaptic loss and neuronal cell death primarily affect brain regions involved in memory and cognitive function. In Parkinson’s, the damage affects motor regions of the brain due to the death of dopaminergic neurons in the substantia nigra which disrupt motor control.
Overall, neuronal cell death-induced synaptic loss and network disruption can significantly impact brain function, resulting in a wide range of cognitive, motor and sensory deficits.
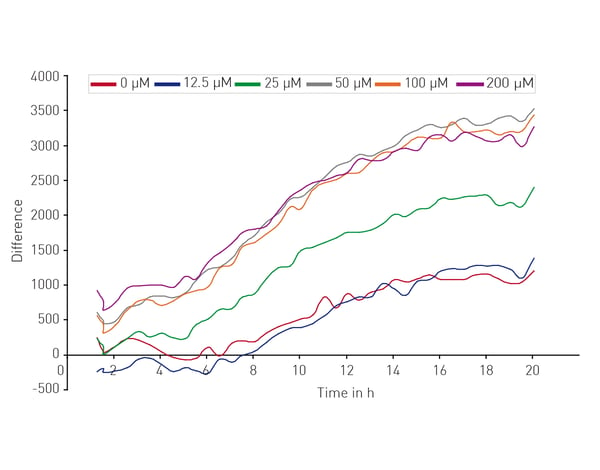
Microplate readers can be used in different ways to look at the impact of neuronal cell death on neurodegenerative disease. They are an effective way to measure cell viability and to perform cytotoxicity assays. In the application note “High throughput method for dynamic measurements of cellular viability using a BMG LABTECH microplate reader” the cell viability of cerebellar granule cells, the smallest neurons found in the human brain, was measured using a propidium iodide assay. Kinetic measurements of fluorescence over 24 h allowed for determination of lag times and death rates (Fig. 2). 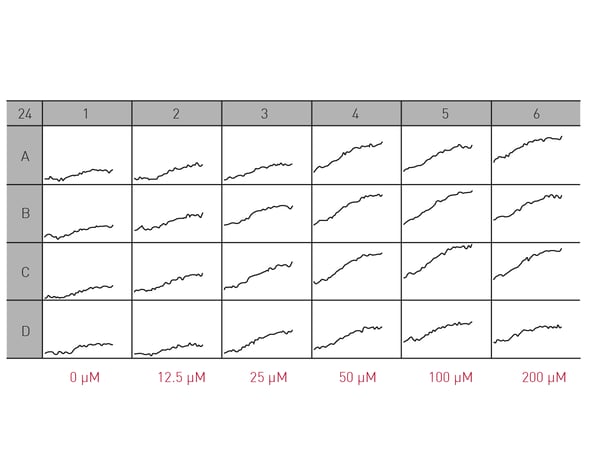 The propidium iodide assay is a useful tool for measuring neuronal viability. In this case, it allowed a complete analysis of the dynamics of a response to glutamate which induced cell death in a dose-dependent manner (Fig. 3).
The propidium iodide assay is a useful tool for measuring neuronal viability. In this case, it allowed a complete analysis of the dynamics of a response to glutamate which induced cell death in a dose-dependent manner (Fig. 3).
 In practice, either absorbance or fluorescence measurements can be used to assess cell viability and cytotoxicity in neuronal cell cultures. These assays use specific dyes that indicate the intactness of cells or other reagents that measure the release of intracellular components. Assays using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromde) or resazurin, for example, can provide quantitative measurements of cell viability or measure the release of intracellular components which are often reduced in dying or dead cells.
In practice, either absorbance or fluorescence measurements can be used to assess cell viability and cytotoxicity in neuronal cell cultures. These assays use specific dyes that indicate the intactness of cells or other reagents that measure the release of intracellular components. Assays using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromde) or resazurin, for example, can provide quantitative measurements of cell viability or measure the release of intracellular components which are often reduced in dying or dead cells.
Microplate readers equipped with fluorescence detection can be used to measure the levels of reactive oxygen species in neuronal cells. Fluorescent probes such as 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA) can be used to measure intracellular levels of ROS. These probes can be oxidized by ROS leading to a fluorescent signal that can be read by the microplate reader. You can read more about the detection of ROS in the BMG LABTECH blog Reactive oxygen species detection.
Microplate readers can also be used to assess the antioxidant or protective effects of various compounds or molecules against ROS activity. By quantifying the reduction in absorbance or fluorescence signals from the scavenging of free radicals, the efficacy of antioxidant interventions can be assessed. In the application note “Comparison of thioredoxin activity in cortical neurons and glial cells using a BMG LABTECH microplate reader” researchers looked at the protective effects of thioredoxin against ROS in glial cells and neurons. Thioredoxin activity assays were readily performed on a BMG LABTECH reader and revealed an almost four-fold higher protective effect of thioredoxin in neurons versus glial cells (Fig. 4). Measurements of thioredoxin activity were made by monitoring the absorbance at 405 nm.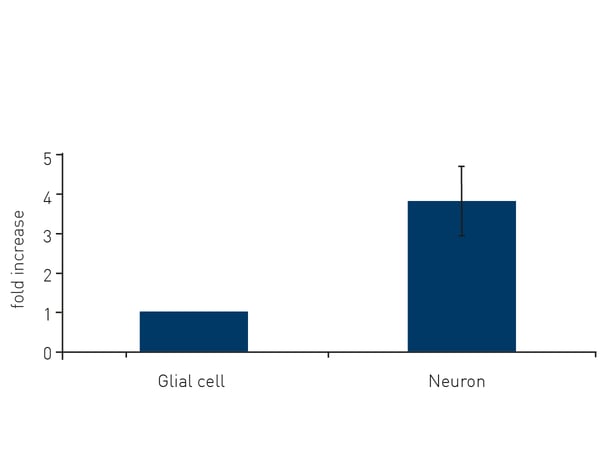 Microplate readers can be used to assess apoptotic cell death in neuronal cell cultures by measuring specific markers or events associated with apoptosis. For example, assays of caspase activity utilize fluorogenic substances that are cleaved by active caspases, which can indicate the activation of cell death pathways.3 Fluorescence-based assays for DNA fragmentation or annexin V binding, which occur during apoptosis, can also be measured on a microplate reader. You can read more about assay selection for apoptosis in the BMG LABTECH blog Apoptosis – which assay should I use?
Microplate readers can be used to assess apoptotic cell death in neuronal cell cultures by measuring specific markers or events associated with apoptosis. For example, assays of caspase activity utilize fluorogenic substances that are cleaved by active caspases, which can indicate the activation of cell death pathways.3 Fluorescence-based assays for DNA fragmentation or annexin V binding, which occur during apoptosis, can also be measured on a microplate reader. You can read more about assay selection for apoptosis in the BMG LABTECH blog Apoptosis – which assay should I use?
As mentioned earlier, mitochondrial dysfunction is implicated in neurodegenerative disease and can contribute to neuronal cell death. Microplate readers can be readily used to measure membrane potential, ATP production, or ROS generation a topic which is covered in more detail in the BMG LABTECH blogs Mitochondrial toxicity: measurement and applications and Mitochondrial dysfunction and neurodegenerative disease.
In addition, high throughput screening applications are well suited to microplate readers making them valuable tools for studying the impact of neuronal cell death on neurodegenerative disease. They can facilitate the screening of potential therapeutic compounds, the assessment of disease models, and the elucidation of the underlying mechanisms of disease.
Different therapeutic strategies are underway to target neuronal cell death. Researchers are looking at neuroprotective agents to protect neurons from degeneration and cell death. These agents often target pathways or molecules linked to oxidative stress, inflammation, or protein misfolding. Neurotrophic factors are being pursued as ways to promote the growth, survival, and function of neurons. Some studies are looking at introducing neurotrophic factors directly to the brain or using gene therapy techniques to enhance their production. Inhibitors of specific steps in apoptotic pathways as well as targets for protein misfolding and aggregation are being actively investigated as interventions.
Future directions for research on neuronal cell death are likely to focus on several areas. As more information is gleaned about the steps that make up the different cell death pathways, new targets will emerge for interventions. Precision medicine and personalized therapies will benefit from developments in biomarkers that can stratify patients into subgroups of more closely related individuals. Neuroinflammation is a common feature of many neurodegenerative diseases. Researchers are looking at ways to use immunotherapies to modulate the immune response and reduce neuroinflammation. This may involve targeting specific immune cells, cytokines or signaling pathways involved in neuroinflammatory processes. Gene therapy and RNA-based therapeutics offer promising new avenues to target neurodegenerative disease. Improvements to gene and RNA delivery systems to the brain are needed to target specific regions of the brain. Other areas for refinement include enhancing efficacy and minimizing side effects. Gene editing technologies such as CRISPR-Cas9 should in time provide precise ways to correct gene-causing mutations linked to neurodegenerative disease. Combinations of some of the interventions described here may also deliver new options for patients.
The PHERAstar FSX was specifically conceived for screening campaigns and is your go-to reader for high-performance high-throughput screening.
Both the VANTAstar® and CLARIOstar Plus allow for wavelength flexibility and include Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities. In addition, the Omega series, CLARIOstar Plus and PHERAstar FSX microplate readers come with on-board injectors that can offer the very best options for detection at the time of injection.
BMG LABTECH’s Omega series of readers are a preferred choice for RT-QuIC aggregation assays as they have the robustness to withstand extensive and prolonged shaking.
Collectively, these multi-mode readers combine high performance with miniaturized assays, short measurement times, and offer considerable savings on materials and other resources.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
The disruption of mitochondrial function is linked to neurodegenerative diseases. Find out how microplate readers advance research into mitochondrial dysfunction and neurodegenerative diseases.
The way proteins misfold and aggregate is linked to many neurodegenerative diseases. Find out how microplate readers can help advance research into protein misfolding.
Alpha-synuclein is a key protein involved in neurodegenerative diseases like Parkinson’s. Find out how microplate readers can help advance alpha-synuclein research.
Amyloids are thought to play a crucial role in neurodegeneration. Find out how microplate readers help advance amyloid research.
The tau protein plays a role in many neurological diseases and disorders. Find out about neuronal toxicity induced by tau and how microplate readers can aid tau research.