Introduction
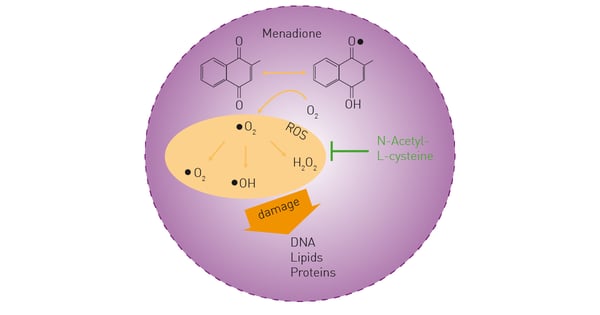
Reactive oxygen species (ROS) are chemically reactive derivatives of molecular oxygen that arise from aerobic metabolism1. ROS play an important role in cell signaling and gene expression2. However, if ROS accumulate at high levels inside the cell, they can also damage DNA, RNA, proteins and lipids (fig. 1). High levels of ROS can occur due to oxidative stress and pose an overall threat to cellular health3. ROS are also associated with aging, apoptosis or necrosis, and are implicated in diabetes, vascular diseases, pulmonary disorders, inflammatory diseases, and cancer4.
There are many intracellular sources of ROS, including mitochondria and NADPH oxidases3. ROS detection assays are used in drug screening campaigns to determine the effects of compounds from chemical libraries on enzymatic reactions (e.g. NADPH oxidase) and to study the effects of antioxidant therapies. ROS typically have a short half-life. H2O2 is an exception and has the longest half-life of all known ROS, which makes it a good marker of oxidative stress and well suited for ROS detection.
Menadione is a compound that interrupts the electron transport chain in mitochondria, producing large amounts of ROS in cultured cells (fig. 1). Hence, it is often used as a control reagent in ROS detection. N-Acetyl-L-cysteine (NAC) functions as an antioxidant and shows an inhibitory effect on ROS generation.
Assay Principle
The Promega ROS-Glo™ assay can be used for ROS detection in biochemical and cell-based applications. Cells are therefore incubated under mammalian cell culture conditions with the test compounds and the ROS-Glo H2O2 substrate. The substrate reacts with available H2O2 and generates a luciferin precursor for ROS detection (fig. 2). After addition of the ROS detection solution, the D-cysteine contained in the solution enables the conversion of the luciferin precursor to luciferin. Finally, this luciferin is converted by the Ultra-Glo™ luciferase and a luminescence signal is generated for ROS detection.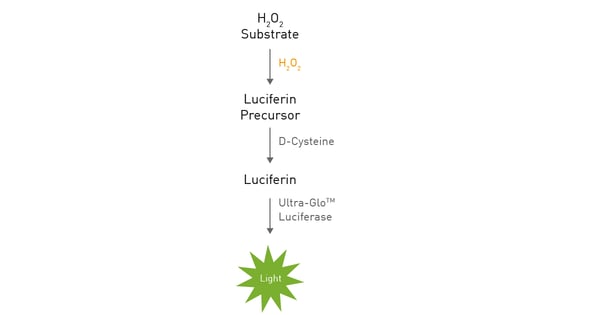
Materials & Methods
- 384-well plate, tissue culture (TC)-treated, transparent(#CLS4681, Corning)
- HeLa cell line (ACC 57, DSMZ)
- DMEM phenol red-free, high glucose, with 10% FBS, 2 mM glutamine, and 1% pen/strep (all Thermo Fisher)
- Incubator with 37°C, 5% CO2 and humidity control
- ROS-Glo (#G8820, Promega)
- 384-well plate, white, med.-binding (#781075, Greiner Bio-One)
- VANTAstar microplate reader (BMG LABTECH)
Experimental procedure
ROS induction with menadione
Cells were seeded in transparent 384-well plates with 2,000 cells per well in 80 µL medium (six replicates each). After cells were attached overnight, 48.4 µL medium were aspirated and 31.6 µL medium remained in the wells. Menadione (10, 50 or 100 µM) or vehicle and ROS-Glo substrate solution were added in a total volume of 8.4 µL to the remaining medium. Cells, ROS-inducer and substrate solution were incubated for 2 h at 37°C and 5% CO2.
40 µL supernatant were transferred to a white 384-well plate for ROS detection with the ROS-Glo assay. The ROS-Glo detection solution was prepared according to the manufacturer’s instructions and 40 µL were added to each well and incubated for 20 min at room temperature until luminescence was read for ROS detection.
ROS inhibition with N-acetyl-L-cysteine
ROS inhibition studies were performed using, the same steps described for the ROS induction experiments. In addition to 50 µM menadione, different concentrations of NAC (12.5, 25, 50, 100, 200 or 400 µM) or vehicle were administered with the substrate solution. Luminescence was measured at the following settings for ROS detection:
Instrument settings
|
Optic settings
|
Luminescence, endpoint
|
|
|
No filter
|
||
|
General settings
|
Interval time
|
1.0 s
|
|
Settling time
|
0.1 s
|
|
|
Focal height
|
11.5
|
|
|
Dynamic Range
|
EDR
|
|
The Enhanced Dynamic Range (EDR) function available on the VANTAstar ensures that each well is read with optimal gain to allow the maximum dynamic range and highest sensitivity. EDR also avoids signal saturation.
Furthermore, the VANTAstar automatically applies the ideal focal height and moves the aperture spoon into the light path. This reduces crosstalk light from non-target wells during luminescence-based ROS detection.
Results & Discussion
ROS induction with menadione
Menadione treatment of HeLa cells resulted in a concentration-dependent increase in ROS (fig. 3). Cell-free assays, which contained solely medium and the ROS inducer menadione also showed a concentration-dependant increase in ROS, which was however lower compared to ROS detection for experiments with cells. This difference arises since some compounds undergo reactions in cell culture medium to produce H₂O₂ independent of cells (abiotic ROS production). In addition, cultured cells have a strong capacity to eliminate H2O2. Thus, ROS production in the preparations with cells was assumed to be higher than the final values obtained in ROS detection since part of the produced H₂O₂ was already degraded by the cells during the incubation period.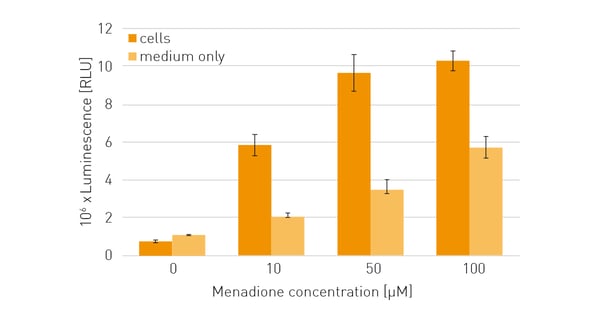
ROS inhibition with N-acetyl-L-cysteine
The ROS-inducing effect of the menadione treatment was eliminated at increasing concentrations of NAC (fig. 4). The luminescence-based ROS detection signal, which reports ROS detection, was reduced to less than the control value (cells with medium only) with the addition of 400 µM NAC. This suggests further inhibition of cellular ROS production by NAC to levels less than those that cells would normally generate without a menadione treatment.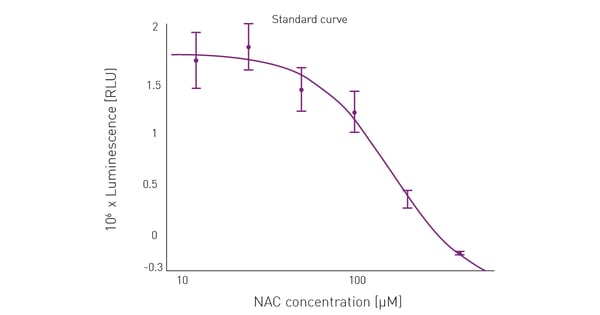
An EC50 of 161.5 µM was determined for NAC using BMG LABTECH’s MARS data analysis software.
Conclusion
The VANTAstar delivers automatic crosstalk reduction by means of an aperture spoon and automatic adjustment to the optimal focal height for ease of use. In addition, the EDR applies the ideal gain to each well and ensures the maximum dynamic range and highest sensitivity while avoiding detector saturation.
ROS detection was successfully performed with the VANTAstar. The high sensitivity of the ROS-Glo assay allowed the ROS detection procedure to be miniaturized for use on 384-well microplates. The homogeneous format of the assay enables easy handling for ROS detection even in cell-based experiments.
References
- Halliwell, B. & Gutteridge, J. M. C. Free Radicals in Biology and Medicine 5th edn, Oxford Univ. Press (2015)
- Lennicke, C., Cochemé, H.M.. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol Cell. (2021) 81(18):3691-3707. doi: 10.1016/j.molcel.2021.08.018. PMID: 34547234.
- Holmström & Finkel. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nature Reviews, Molecular Cell Biology (2014) (15) 411.
- Sharifi-Rad, M. et al., Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol. (2020) 11:694. doi: 10.3389/fphys.2020.00694. PMID: 32714204; PMCID: PMC7347016.