Introduction
High-throughput cell viability assays are broadly used in screening experiments, e. g. for compound toxicity screens or to identify compounds that selectively kill cancer cells. Other common applications include screenings for environmental toxins or simple normalizations of other readouts to the total cell number. There are numerous viability assay kits available which allow quantifying cellular viability using absorbance (Abs), fluorescence (FI), or luminescence (Lum) readouts in a microplate reader. In this application note, we aimed to compare three popular representatives (Vybrant® MTT, alamarBlueTM, and CellTiter-Glo®) in terms of their usability, as well as the lower and upper limit of detection, to support you in the selection of the optimal viability assay for your individual application.
Assay Principle
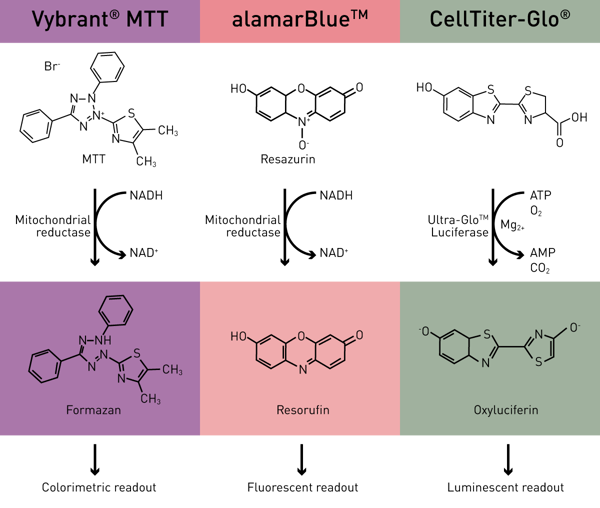
All assays rely on an active respiratory chain, which can only be found in viable cells. The Vybrant MTT viability assay uses the reduction potential of living cells resulting in the turnover of MTT to a formazan derivative intracellularly (colorimetric), while the alamarBlue viability assay leads to the turnover of resazurin to resorufin extracellularly (colorimetric or fluorescent). The CellTiter-Glo viability assay requires ATP in order to generate Oxyluciferin.
Materials & Methods
- TC-treated 96-/384-well plate, transparent (#CLS3595, #CLS4681, Corning)
- TC-treated 96-/384-well plate, black, clear bottom (#655866, #781866, Greiner Bio One)
- TC-treated 96-/384-well plate, white (#655073, #781073, Greiner Bio One)
- WT HeLa cell line
- DMEM phenol red-free with 10% FBS, 2 mM glutamine, and 1% pen/strep, all Thermo Fisher
- Incubator with 37°C, 5% CO2 and humidity control
- Vybrant® MTT (#V-13154, Thermo Fisher)
- AlamarBlueTM (#DAL1025, Thermo Fisher)
- CellTiter-Glo® 2.0 (#G9241, Promega)
- VANTAstar® microplate reader (BMG LABTECH)
Experimental procedure
Cell culture:
On the day before the measurement, precultured HeLa cells were seeded at densities of 25-25,000 cells/well in 50 µL in 384-well plates and 50-50,000 cells/well in 100 µL in 96-well plates. Seeded cells were incubated overnight.
Viability assays:
All viability assays were performed according to the manufacturers’ protocols including recommended volumes and incubation times.
Vybrant MTT viability assay
Medium was replaced with fresh medium including MTT reagent (44 µL were added for 384-well plates) and plates were incubated for 4h. After the addition of the SDS stop solution (40 µL for 384-well plates), the cells were homogenised in the plate by mixing and incubated for 18h.
AlamarBlue viability assay
Medium was replaced with fresh medium containing alamarBlue reagent and incubated for 4h. 3% SDS was added to stop the reaction before reading.
CellTiter-Glo viability assay
Medium was discarded and replaced with fresh medium. CellTiter-Glo reagent was added to the wells. Plates were shaken for 2 min at 200 rpm.
Instrument settings
| Absorbance, Vybrant® MTT | ||
| Optic settings | Absorbance, endpoint | |
| Wavelength | 570 nm | |
| General settings | Number of flashes | 22 |
| Settling time | 0.5 sec (96) 0.2 sec (384) |
|
| Fluorescence intensity, alamarBlueTM | ||
| Optic settings | Fluorescence intensity, endpoint | |
| Monochromator | Ex 545-20 Auto dichroic Em 600-40 |
|
| General settings | Number of flashes | 20 |
| Settling time | 0.5 sec (96) 0.1 sec (384) |
|
| Start settings | Focus | Automatic adjustment |
| Dynamic Range | EDR | |
| Luminescence, CellTiter-Glo® |
||
| Optic settings | Luminescence, endpoint | |
| Filters No filters | No filters | |
| General settings | Measurement interval time | 0.8 sec |
| Start settings | Focus | Automatic adjustment |
| Dynamic Range | EDR | |
Results & Discussion
Comparison of viability assay sensitivity
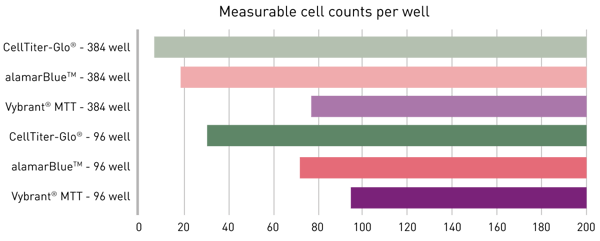
The limit of detection (LOD) of an assay is defined as the concentration at the mean blank value plus three times the standard deviation of the blank (mean blanks + 3*SDblank). As displayed in figure 2, CellTiter-Glo provided the lowest LODs, followed by alamarBlue and Vybrant MTT. Measurements in the 384-well format generally allowed the detection of lower cell numbers. This benefit can be mainly attributed to the increased substrate/cell ratio applied here.
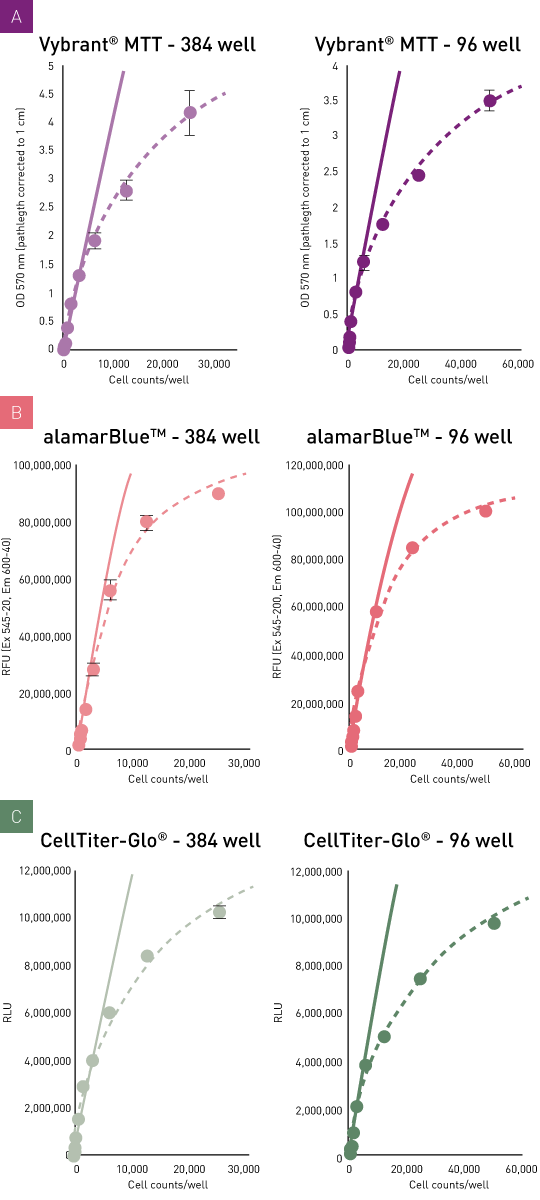
Comparison of viability assay dynamic range
A distinct signal increase as a function of cell number was observed in all assays. The linear relation, which is observed for the largest concentration range, does however dissipate at high cell concentrations (fig. 3A, B, C). This effect is probably primarily attributed to limited substrate availability. Substrate turnover/cell strongly depends on cell type and cell culture conditions such as FCS availability. In this setting, the relatively active HeLa cells resulted in a high turnover/cell, leading to low LODs but at the same time to an incipient saturation effect at high cell concentrations. This points out that the manufacturers’ recommendation is based on a general guideline, but these parameters should be checked and, if needed, adapted to your individual experimental conditions in advance. Possible parameters may include the adjustment of the overall cell number, the ratio of cells to the amount of reagent as well as the adjustment of respective incubation times.
Conclusion
The VANTAstar microplate reader provides a perfect detection platform to cover a broad range of cell viability assay read-outs ranging from Abs to FI to Lum with high sensitivity. Its monochromator and spectrometer provide full flexibility in wavelength selection and accordingly in the choice of the viability assay. The use of EDR and automatic focus adjustment make a preliminary gain or focus optimization superfluous. While CellTiter-Glo allows detecting <10 cells/well with an easy add and read procedure, alamarBlue offers the option to choose between FI or Abs detection and does also allow kinetic monitoring since cells don’t have to be lysed. AlamarBlue also constitutes the most affordable option, followed by Vybrant MTT, and finally CellTiter-Glo.