Introduction
Drugs are characterized by their equilibrium constant KD which describes the affinity of the compound to its target. It refers to half the ligand concentration of saturated binding. However, this dissociation constant only applies to the equilibrium state and neglects the importance of the speed of association and dissociation. The optimal kinetic binding of a drug differs with the target of interest and related disease. On the one hand, slow dissociation constants can be beneficial to increase the duration of drug action (as for example Tiotropium for COPD treatment - Disse et al. Life Science, 1999). On the other hand, it has been hypothesized for specific targets that fast dissociations could decrease on target side effects (Kapur & Seeman, J Psychiatry Neurosci, 2000). Here, we present the determination of the association and dissociation rate of Spiperone-d2 binding to the dopamine receptor D2 (D2R), a G-protein coupled receptor (GPCR) and target of antipsychotic drugs.
Assay Principle
The binding of Spiperone-d2 to D2R was monitored using cells with terbium cryptate labelled receptor and d2-fluorophore-labelled Spiperone.
The terbium cryptate is a fluorophore with a long lifetime acting as a donor. It transfers energy to the Spiperone-associated d2 in case the ligand is bound to the receptor. Thus the interaction of ligand with receptor is described by the ratio of acceptor to donor emission signal.
Materials & Methods
- Tag-lite Dopamine D2 labelled cells (Cisbio Bioassays, #C1TT1D2)
- Spiperone-d2 (Cisbio Bioassays, #L0002RED)
- Tag-lite labelling medium (Cisbio Bioassays, #LABMED)
- 384 well plate (small volume, Greiner #784075)
Experimental Procedure
The reaction was performed according to the instructions for Cisbio’s ligand binding protocol. Briefly, in a total reaction volume of 20 µl, 3.700 donor-labelled cells were incubated with different concentrations of the d2-labelled ligand Spiperone. The binding was monitored in real-time by the PHERAstar using the instrument settings outlined below.
Instrument Settings
| Optic settings | Time resolved fluorescence, plate mode kinetic, simultaneous dual emission | |
| Optic module: | HTRF | |
| General settings
|
Number of flashes: | 40 |
|
Settling time: |
0 s |
|
|
Integration start: |
60 µs |
|
|
Integration time: |
400 µs |
|
| Kinetic settings |
Number of cycles: | 60 |
| Cycle time: | 60 s | |
| Incubation | None | |
Results & Discussion
a) Determine kon and koff by association experiments on the PHERAstar
Kinetic measurement of ligand binding for kon/koff determination
The binding of a ligand to its receptor is not static but is defined by the rate at which the ligand binds and by the rate it leaves its interaction partner. These dynamics are described by the on-rate (kon, how many ligand molecules bind the receptor in a minute), and by the off-rate (koff, which portion of ligand-receptor pairs dissociate in a minute). In order to determine both rates, the ligand Spiperone-d2 was added to receptor-expressing cells and monitoring of the HTRF signal was started immediately (Fig. 2).
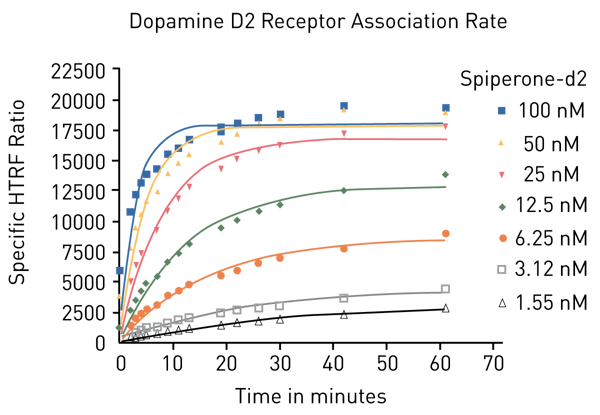 Based on the association measurements, the time at which half of the maximum binding was reached, was calculated for each ligand concentration (t1/2 association). According to the following relation, an observed association rate was calculated (kobs). Alternatively, kobs can be calculated directly with the “one phase association” equation of the GraphPad Prism bioanalysis software.
Based on the association measurements, the time at which half of the maximum binding was reached, was calculated for each ligand concentration (t1/2 association). According to the following relation, an observed association rate was calculated (kobs). Alternatively, kobs can be calculated directly with the “one phase association” equation of the GraphPad Prism bioanalysis software.
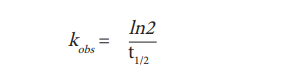
Table 1: Calculation of observed association kobs
|
Spiperone-d2 (Nm) |
100 | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.55 | 0 |
| t 1/2 association | 2.11 | 3.34 | 5.56 | 8.83 | 11.02 | 15.4 | 19.35 | 0.273 |
| Kobs (min-1) | 0.33 | 0.21 | 0.12 | 0.08 | 0.06 | 0.05 | 0.04 | 2.54 |
As the binding of Spiperone-Red onto the D2R receptor follows a simple law of mass action model, kobs should increase in a linear manner with labeled ligand concentration (Hill, J Physiol, 1909), and then the observed association rate kobs is defined as the following linear equation:
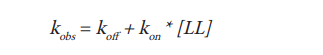
The observed association rate kobs increases linearly with an increase of the ligand whereas the association rate (kon) and the dissociation rate (koff) are constants. Plotting the ligand concentration against kobs allows determination of the kon and koff rate (Fig. 3). The data are consistent with a straight line indicating that binding follows the law of mass action and that the models can be applied.
 Having determined kon and koff, the equilibrium constant is calculated as follows:
Having determined kon and koff, the equilibrium constant is calculated as follows:
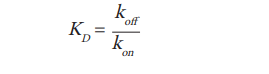
Table 2: Binding kinetics determined by association experiments
|
koff |
0.0354 min-1 |
| kon | 0.00346 nM-1 * min-1 |
| KD | 10.23 nm |
Comparison - Classical KD determination at equilibrium
The equilibrium constant KD describes the ligand concentration at which half of the maximum ligand-receptor binding is reached. It is typically determined by measuring ligand binding at binding equilibrium. To this end, the fluorophore-labelled ligand Spiperone-d2 was added at different concentrations to D2-receptor expressing cells. At equilibrium (60 min after ligand addition) HTRF signal was recorded (Fig. 4) and the Spiperone-d2 concentration at which half of the maximum value is reached was calculated to be KD = 8 nM ( 1hour ) (Fig. 4).

b) determine koff by dissociation measurements on the PHERAstar and calculate kon
Kinetic measurement of dissociation for koff determination
In order to measure the dissociation of the fluorophore-labelled ligand Spiperone-d2, it was first incubated at different concentrations with the receptor to allow binding. Dissociation was then initiated by introducing an excess of an unlabelled dopamine receptor agonist (Bromocriptine). Dissociation of the ligand-receptor interaction was monitored by measuring the HTRF signals on a PHERAstar microplate reader (Fig. 5).
The observed decrease of HTRF ratio stems from separation of donor fluorophore from the acceptor fluorophore that precludes energy transfer and is indicative of dissociation of the spiperone-d2 ligand from the D2R receptor.
 The dissociation rate koff can be derived from this experiment by using the following equation.
The dissociation rate koff can be derived from this experiment by using the following equation.
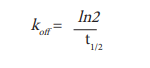 In order to calculate the association rate kon, determination of KD in a steady state experiment is required. To this end, different concentrations of the Spiperone-d2 were incubated with D2R expressing cells (Fig. 6). Half maximal binding (KD) was determined to be 8 nM. The calculated kon (=koff/KD) is accordingly 8.75*10-4 nM-1 min-1 (Table 1).
In order to calculate the association rate kon, determination of KD in a steady state experiment is required. To this end, different concentrations of the Spiperone-d2 were incubated with D2R expressing cells (Fig. 6). Half maximal binding (KD) was determined to be 8 nM. The calculated kon (=koff/KD) is accordingly 8.75*10-4 nM-1 min-1 (Table 1).
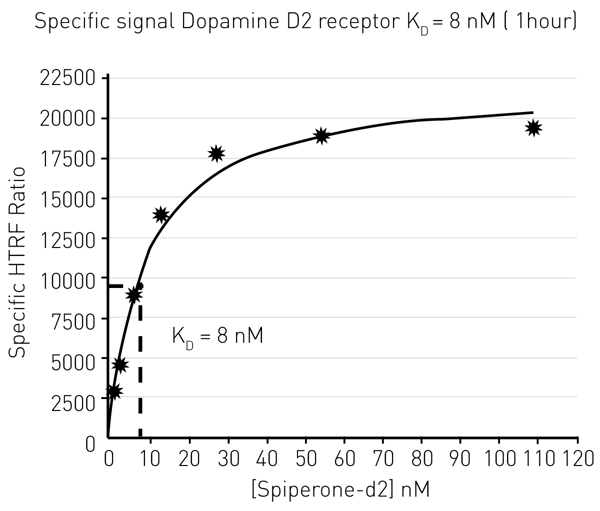 Table 3: Dissociation pharmacokinetic values of Spiperone-d2
Table 3: Dissociation pharmacokinetic values of Spiperone-d2
|
t 1/2 dissociation |
99 min |
| koff | 0.007*min-1 |
| KD | 8nM |
| kon= koff/KD | 0.000875 nM-1 min-1 |
Table 3 summarizes the values obtained during the dis-sociation experiment for Spiperone-d2. Koff is obtained directly from the dissociation experiment and KD from the steady-state experiment while kon is calculated.
Alternative determination of the association rate kon
Another way to calculate the kon rate combines data from this dissociation experiment with data from an association experiment (see complete description in Chapter a). This calculation is based on the fact that actual binding is the sum of speed of binding and release. In case of binding that follows the law of mass action (Hill, J Physiol, 1909), the equation for kon that describes this assumption is as follows:
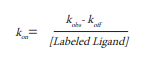
This way the resulting association rate is 0.0032 nM -1 min -1.
Conclusion
Here, the PHERAstar microplate reader was combined with Cisbio’s Tag-lite® assay technology to measure the binding kinetics of Spiperone-d2 binding to the cellular Dopamine D2 receptor.
The homogeneous Tag-lite binding technology combined with the speed of the PHERAstar offer a high temporal resolution that is absolutely required to monitor the binding dynamics and subsequently calculate the on- and off-rates.
Lumi4 is a trademark of Lumiphore Inc.

