Introduction
E. coli are common bacteria found in the gut of humans and animals and normally help to maintain a healthy microbiome. However, some E. coli strains are pathogenic, causing serious disease, such as urinary tract infections, diarrhea, meningitis, and sepsis. Pathogenic E. coli strains are becoming increasingly resistant to most of the antibiotics commonly available today. Therefore, there is a need for new drugs with antibacterial properties or alternative ways to fight these infections.
One attractive alternative to traditional antibiotics is phage therapy. Bacteriophages are viruses with antibacterial properties that specifi cally infect bacteria (causing bacterial cell lysis) without any adverse effects to humans or animals.
In this study, a novel bacteriophage (APTC-EC-2A) was isolated and characterized from hospital wastewater. APTC-EC-2A shows promising antibacterial properties and activity against several antibiotic resistant strains of E. coli.1
However, E. coli (and many other bacteria) can also form biofilms, which are commonly more resistant to antimicrobials.2 To assess the antibacterial properties of the phage APTC-EC-2A towards bacterial biofilms, crystal violet biofilm mass quantification and Live/Dead bacterial viability assays were employed.
Assay Principle
The crystal violet biofilm assay, in microplates, was developed over 30 years ago, with several modifications and improvements since then.3 Essentially, a bacterial biofilm is grown on the bottom of a 96-well plate and then treated with varying concentrations of phages (or other antimicrobial). The remaining adherent biofilm is then stained with crystal violet. Unbound dye is washed from the well and the bound dye is dissolved by addition of 30% acetic acid in each well. The absorbance of the supernatant at 595 nm is then proportional to the mass of the biofilm (fig. 1).
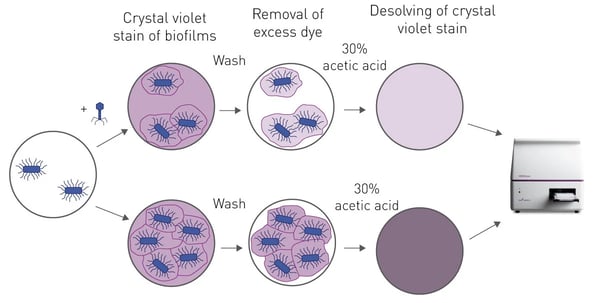
Materials & Methods
- Microplate (96-well clear polystyrene, Costar)
- BMG LABTECH Microplate reader
- Crystal violet, Sigma Aldrich
- LIVE/DEAD™ BacLight™ Bacterial Viability Kit, Thermo Fisher Scientific
Experimental procedure
Biofilm formation
Briefl y, a 1 McFarland unit of E. coli XL10 suspension in saline was diluted 1:15 into Luria-Bertani (LB) broth. In a 96-well plate, a column of six wells was filled with only LB broth, as the negative control, the rest were filled with 150µL of bacterial suspension. The plate was incubated for 48 h at 37 °C to allow biofilm development and washed twice with sterile 1 x PBS.
Phage application
The phage in LB broth was applied in 200 µL per well at concentrations of 5 x 108 plaque forming units (pfu) per mL and 5 x 109 pfu/mL. LB broth only was used as negative control and LB with bacterial suspension as positive control. The plate was incubated at 37 °C for 24 h.
Crystal Violet Staining
After phage treatment, the bacterial wells were washed with sterile 1 x PBS to remove all the planktonic bacteria in solution. The plate was stained with 200µL/well 0.1 % crystal violet for 15 min. The stained plates were rinsed with distilled water and left to dry overnight. The remaining crystal violet stain in each well was dissolved by adding 30 % acetic acid and absorbance was measured at 595 nm. The relative biomass was determined by normalizing to the absorbance of the positive control. This procedure was repeated with antibiotic resistant ED, PC and 378 E. coli strains.
Instrument settings
| Optic settings | Absorbance, endpoint | |
| Discrete wavelength | 595-10 nm | |
| General settings | Number of flashes | 22 |
| Settling time | 0.5 sec | |
Results & Discussion
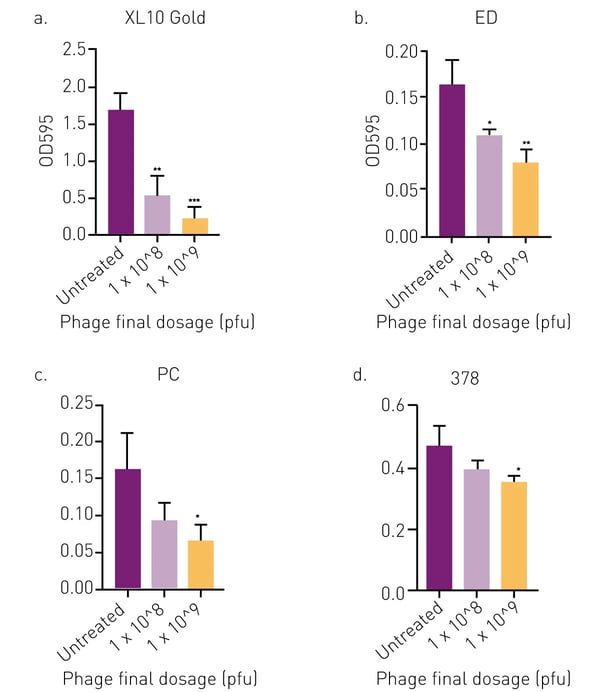
The results clearly show that the tested phages possess antibiofilm properties with a dose dependent decrease in biofilm biomass as phage dosage increases. Phage APTC-EC-2A, at a final dosage of 1×109 pfu, significantly reduced the biomass of XL10 Gold, ED, PC and 378 strains compared to the no-treatment control (fig. 2). Reduction of biofilm biomass was up to 53 % (range 16–54 %) when treated with APTC-EC-2A at the final dosage of 1×108 pfu; and up to 70 % (range 27–70 %) when treated with APTC-EC-2A at the final dosage of 1× 109 pfu.
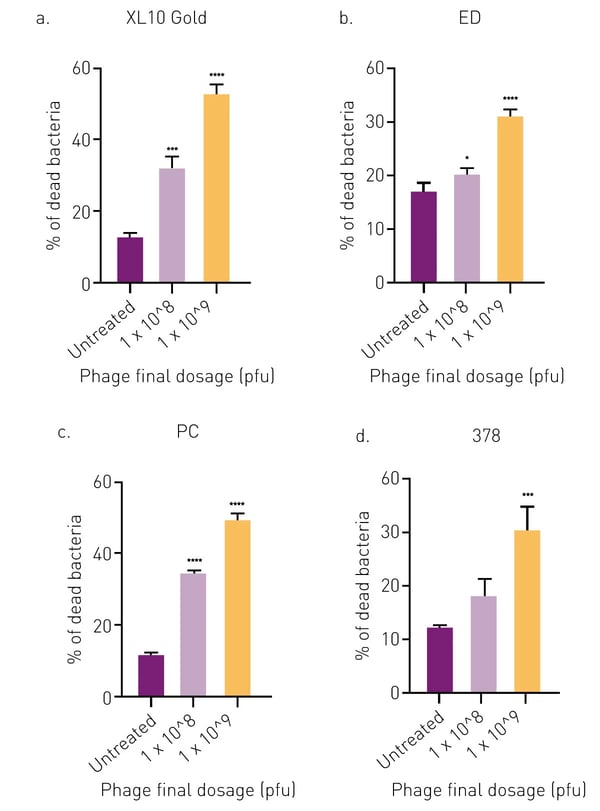
The LIVE/DEAD BacLight Bacterial viability kit was used to determine the percentage of live (SYTO9, green) and dead (PI, red) E. coli cells after APTC-EC-2A phage treatments. There was a statistically significant increase in the percentage of dead cells of E. coli XL10, ED and PC in APTC-EC-2A-treated biofilms as compared to the untreated control at both phage concentrations. However, for E. coli 378, only the highest concentration of APTC-EC-2A showed a significant percentage of cell death and antibacterial properties compared to the control (fig. 3).
Conclusion
This research identifies a new bacteriophage with antibacterial properties against antibiotic resistant E. coli. Its broad range lytic activity and stability, under a wide pH and temperature range, makes it attractive for further preclinical and clinical development.
The BMG LABTECH plate reader range has, for many years, been used extensively for bacterial growth assays. Kinetic growth curves can be determined within the reader, with incubation and long term, heavy-duty shaking as required. All instruments are equipped with a solid-state CCD spectrometer for all absorbance measurements from 220-1000 nm. This makes them ideal measurement platforms for the determination of drugs and other pharmaceuticals with antibacterial properties.
References
- Hon,K., et al. APTC-EC-2A: A Lytic Phage Targeting Multidrug Resistant E. coli- Planktonic Cells and Biofilms. Microorganisms, 2022, 10, 102.
- Staneva, A.D., et al. Antibiofouling Activity of Graphene Materials and Graphene-Based Antimicrobial Coatings. Microorganisms 2021, 9, 1839.
- Stepanovic, S., et al. A modified microtiter-13 plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods, 40 14 (2000) 175.

