Introduction
Induced pluripotent stem cells (iPSC) are among the most physiologically relevant models available for use in drug screening1. This utility includes the use of iPSC to discover drug reactions which, if undiscovered, could disrupt drug approval at late stages of approval2.
Here we described the use of iPSC cardiomyocytes in a multiplex platform suitable for detection with a microplate reader.
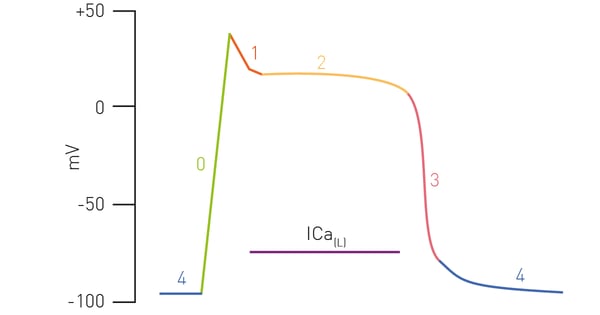
We have previously seen how bioluminescent sensors from 490 Bio Tech can be used to monitor cell metabolism3. Here we see how this can be easily multiplexed with other readouts in iPSC cardiomyocytes. Figure 1 is a depiction of the cardiac action potential that highlights the role of calcium flux.
Assay Principle
Rhod-4 dye exhibits high fluorescence when associated with calcium. This was employed to monitor intracellular calcium in iPSC cardiomyocytes as a readout for effects on contraction, which was multiplexed with iPSC cardiomyocyte’s metabolism assessment.
Materials & Methods
- 384-well, white, clear-bottom plates (Greiner)
- iCell iPSC cardiomyocytes
- LiveLightTM Toxicity Assay Mix (490 Bio Tech)
- Rhod-4 Calcium Assay Kit (Abcam)
- CLARIOstar® with ACU
Cell culture
iPSC cardiomyocytes were plated at 5,000 cells/well in a 384-well plate coated with gelatin. Cells were grown at 37 °C and 5% CO2 for 10 days until beating was observed to be present and consistent within each well. IPSC cardiomyocytes were transfected with LiveLight Toxicity Assay Mix using 0.01 ng DNA/cell and further incubated for 24 h to allow uptake and continuous bioluminescent expression. Cells were transferred to the CLARIOstar and atmospheric conditions were maintained throughout the assay using the ACU.
Measuring immediate effects of isoproterenol
At 24 h post transfection the iPSC cardiomyocytes were challenged with 1 uM isoproterenol to trigger calcium release. Immediately after challenge the iPSC cardiomyocytes were further treated with the Rhod-4 Calcium Assay Kit. Fluorescent readings were obtained at 0 and 1 h after the treatment.
Instrument settings
|
Optic settings
|
Fluorescence intensity, well mode
|
|
|
Monochromator
|
Ex 540-15 nm
Em 590-20 nm |
|
|
Gain
|
2600
|
|
|
Focal height
|
2 mm
|
|
|
General settings
|
Optic settings
|
Bottom Optic
|
|
Kinetic settings
|
Number of intervals
|
1000
|
|
Interval time
|
0.01 s
|
|
|
Incubation settings
|
Target temperature
|
37°C
|
|
Target CO2
|
5%
|
|
Measuring effects of isoproterenol on cell metabolism
IPSC cardiomyocytes’ metabolism was tracked based on the applied Live Light Toxicity Assay Mix. Continuous luminescence readings were obtainable for all time points throughout the 24 h assay.
Instrument settings
|
Optic settings
|
Luminescence, plate mode
|
|
|
Filter(s)
|
None
|
|
|
Gain
|
3500
|
|
|
Focal height
|
11 mm
|
|
|
General settings
|
Optic settings
|
Top Optic
|
|
Kinetic settings
|
Number of intervals
|
25
|
|
Interval time
|
1 hour
|
|
|
Measurement Interval Time
|
1.00 s
|
|
|
Incubation settings
|
Target temperature
|
37°C
|
|
Target CO2
|
5%
|
|
Results & Discussion
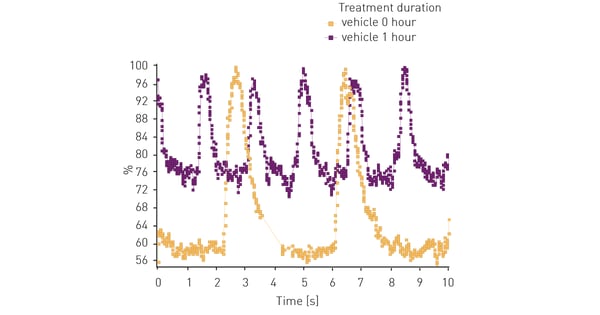
Figure 2 shows representative results of iPSC cardiomyocyte beating activity as measured by intracellular calcium. Without treatment iPSC cardiomyocytes exhibit slight increase in beats per minute (bpm) over time.
At the same time iPSC cardiomyocytes were assessed for the effect treatment with isoproterenol, shown in Figure 3. The results show the expected inotropic effect.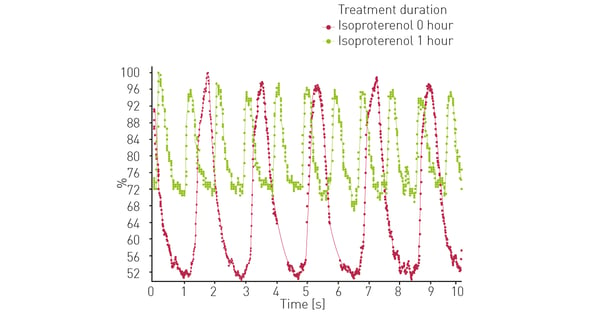
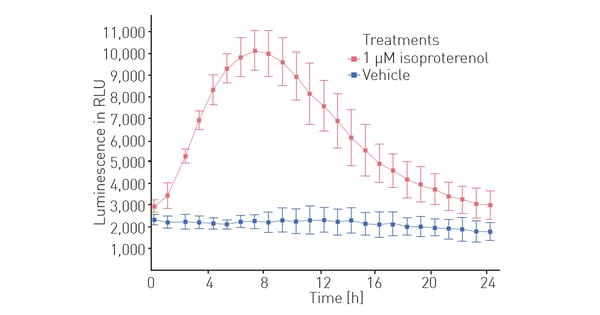
IPSC cardiomyocytes transfected with LiveLight Toxicity mix allowed us to also assess the isoproterenol effects on cell metabolism over a 24-hour period (Fig. 4). The results indicate that treatment with isoproterenol increases cellular metabolism, peaking at 7 hours.
Conclusion
We present the successful multiplexing of calcium flux and cell metabolism measurements using a microplate reader. The CLARIOstar with ACU thereby not only enables incubation and analysis of the living cells in the same instrument, but also detection of two assays in a multiplex approach. The use of iPSC cardiomyocytes represents a physiologically relevant platform that can be used for screening on and off target effects of drug candidates.
References
- Ebert, AD & Svendsen, CN. Human stem cells and drug screening: opportunities and challenges. Nat. Rev. Drug Discov. (2010) 9: 367-372.
- Wang, X et al. Potential Applications of Induced Pluripotent Stem Cells for Cardiovascular Diseases. Curr. Drug Targets (2019) 20: 763-774.
- Halter, K & Close, D. Autobioluminescent cells report real-time changes in cellular metabolism in response to pharmacological challenges. BMG LABTECH, AN 344