
PHERAstar FSX
Powerful and most sensitive HTS plate reader
Atherosclerotic vascular disease is one of the leading causes of death globally. Here we explore some of the research options available to scientists to tackle this significant threat to public health.
 Dr Barry Whyte
Dr Barry Whyte
Cardiovascular disease, a group of disorders of the heart and blood vessels, is the number one cause of death across the world. 1,2 Millions of people die each year or find themselves with disabilities due to its effects. One disorder in particular, atherosclerotic vascular disease, a chronic inflammatory disease associated with the formation of arterial plaques, is considered the major cause of cardiovascular diseases.
Microplate readers are useful tools for researchers to investigate the many facets of cardiovascular disease and provide different options for high-throughput research of atherosclerotic vascular disease.
In this blog, we highlight some of the solutions available for researchers to tackle atherosclerotic vascular disease: from fundamental research rooted in ischemia-reperfusion studies and cell-based assays to drug discovery efforts and the early detection of drug-related side effects.
Cardiovascular disease comprises a group of disorders that affect the heart and blood vessels (Box 1). These disorders can arise due to unwanted changes at the molecular level and are significantly impacted by genetics, lifestyle, and environmental effects. Researchers are interested in understanding the mechanisms of disease to facilitate possible interventions.
Atherosclerotic vascular disease is one of the key drivers of coronary heart disease, the most common type of cardiovascular disease found in countries around the world. Here the gradual build up of fatty material on the inside surfaces of blood vessels causes a narrowing of the arteries which makes it difficult for blood to flow through the circulatory system (cardiac ischemia). This type of impairment results in reduced oxygen supply to cells and tissues which may trigger molecular events like mitochondrial dysfunction and oxidative stress. These changes can in turn lead to an inflammatory response and tissue damage.3
The combined effects of mitochondrial dysfunction, oxidative stress and increased inflammation can have profound effects on the formation of plaques in blood vessels in different parts of the body. If a fragment of fatty material breaks away from a plaque, it can cause the vessel to become blocked which may lead to death of the surrounding cells. If such a blockage occurs in the heart, the death of muscle cells may cause a heart attack. Similar blockages in other parts of the body may lead to a stroke (ischemia in the brain), or damage and ischemia in the skin, small intestine, kidney, or other organs.
Researchers are interested in finding ways to mimic the in vivo environment of cardiovascular disease and are using approaches like ischemia-reperfusion studies or cell-based assays to investigate processes like atherosclerotic vascular disease.
Mitochondrial dysfunction can contribute to atherosclerotic vascular disease in several ways. Mitochondria are the energy-producing organelles within cells and are responsible for producing most of the energy required for myocardial contraction. Inefficient or impaired mitochondrial function can lead to an inadequate supply of ATP which may compromise the heart’s ability to circulate blood effectively. Dysfunctional mitochondria can also release mitochondrial DNA and other proinflammatory molecules into the circulatory system that may activate immune responses in the walls of blood vessels. Chronic inflammation can result which may accelerate the formation of plaques and the development of atherosclerotic vascular disease.
Programmed cell death (cellular apoptosis) is another consequence of mitochondrial dysfunction. In this case, the accumulation of dead cells in plaques contributes to plaque instability and increases the likelihood of their rupture. In addition, mitochondrial dysfunction can trigger the accumulation of unfolded proteins in cells due to the unfolded protein response. The buildup of unfolded proteins can promote an inflammatory response and cell stress which exacerbates atherosclerotic vascular disease.
Oxidative stress can arise from changes within a cell and from dysfunctional mitochondria that produce higher levels of reactive oxygen species. Oxidative stress is known to contribute to atherosclerotic vascular disease by increasing inflammation. Increased levels of reactive oxygen species can damage the molecular fabric of the cell including lipids and proteins. Reactive oxygen species promote oxidative stress in the vascular wall which can lead to the oxidation of low-density lipoprotein cholesterol and the initiation of an inflammatory response.
Collectively, these molecular changes can trigger plaque formation and subsequently, atherosclerotic vascular disease.
Multimode microplate readers are ideally suited for the study of cardiovascular diseases such as atherosclerotic vascular disease. Microplate readers can be used to measure markers of oxidative stress like reactive oxygen species (ROS) or lipid oxidation products using fluorescent or colorimetric assays. They can also be used to look at inflammatory markers such as cytokines, adhesion molecules or C-reactive protein in cell culture supernatants or blood samples. Enzyme-linked immunosorbent assays (ELISAs) are often used for this purpose. Furthermore, the availability of incubation and gas control on BMG LABTECH readers equipped with Atmospheric Control Unit (ACU) allows the performance of complex cell-based assays.
Naturally, some of the best ways to look at cardiovascular disease such as atherosclerotic vascular disease involve cell-based assays or ischemia-reperfusion studies. We will discuss each briefly here but these topics are covered in more detail in other articles from BMG LABTECH (referenced in the sections below).
Cell-based assays provide researchers with ways to investigate the cellular and molecular processes that contribute to atherosclerotic vascular disease. They provide a controlled and reproducible environment to study specific aspects of atherosclerotic vascular disease that reflect the complexity of biological systems and metabolic events. Cultured cells can therefore be used to provide insight into the mechanisms of disease and provide model systems for investigation. In addition to apoptosis and oxidative stress, cell-based assays can be used to look at cell viability, proliferation, migration, cell signaling, gene expression, and other types of cell death like necrosis. You can read more about how cell-based assays can be studied using microplate readers in the BMG LABTECH blog Cell-based assays on the rise.
Ischemia refers to the blocking of blood flow to a specific organ, tissue or cell that results in oxygen and nutrient deficiency. Ischemia may occur due to atherosclerotic vascular disease and can also be induced in an experimental system to study its effects. Reperfusion is the restoration of blood flow to the biological system. After a period of acute ischemia, reperfusion may also lead to damage of cells since the rapid increase of oxygen may generate reactive oxygen species. Ischemia-reperfusion studies, which have typically been performed in small animal models, are a useful way to study atherosclerotic vascular disease. Today, in vitro systems represent a valuable alternative that can reveal the consequences of the oxygen and nutrient deficiencies that arise due to myocardial ischemia and the process of recovery that takes place during reperfusion. Ischemia-reperfusion research is discussed in more detail in the BMG LABTECH blog Ischemia Reperfusion Research.
You can also learn more about ischemia reperfusion in the scientific talk and video Simulating ischemia reperfusion injury in vitro as a potential method for drug screening

Drug discovery for the treatment of atherosclerotic vascular disease
Microplate readers play a crucial role in drug discovery and development efforts aimed at treating atherosclerotic vascular disease. In some cases, applications in drug discovery are related to those used in fundamental research. In other cases, they have unique attributes because of the need for high throughput.
Researchers interested in drug development can assess the effects of thousands of compounds from libraries of molecules for their effects on drug targets using high throughput screening. This may involve measuring enzyme activities, looking at receptor binding or using cell-based functional assays on a large scale.
In drug discovery, microplate readers can also be used to support phenotypic assays that mimic the different conditions of disease. In the case of atherosclerotic vascular disease, this may involve assessing factors like endothelial cell dysfunction, inflammation or lipid accumulation. Different endpoints for this type of assay include looking at cell viability, measuring cytokine production or determining the specific levels of lipids at high throughput.
The drug development process might also require dose-response studies, compound profiling as well as hit validation and lead optimization steps which can each benefit from the high-throughput capabilities of a microplate reader (Fig.2).
As in basic research, cell-based assays also play a significant role in drug discovery. In the context of drug discovery, we will consider here a few examples in more detail that involve induced pluripotent stem cells.
Microplate readers are powerful tools to study atherosclerotic vascular disease since they allow researchers to study different parameters of the disease process often at high throughput. Their uses extend from the discovery of new drugs to studies looking for potential adverse drug reactions that might disrupt the drug approval especially at later stages of the drug development process.
In cardiovascular and other areas of research, induced pluripotent stem cells offer significant advantages to drug discovery and development efforts because of their direct physiological relevance.
Microplate readers can be used to measure the functional responses of induced pluripotent stem cells to drug compounds. Examples include the use of calcium-sensitive dyes such as Fluo-2, Fluo-4 or rhodamine which might provide important information on contractility, a key indicator of the performance of cardiac muscle, or electrophysiological parameters.
In the application note Real-time calcium flux measurements in iPSC derived 3D heart tissues researchers used a CLARIOstar® to measure changes in calcium ion levels using the fluorescent dye Fluo-4 in cardiomyocytes, a specific type of induced pluripotent stem cell. The CLARIOstar can measure rapid changes in fluorescent intensity for Fluo-4 measurements (one data point every hundredth of a second) and is ideally suited to measure transient changes in the levels of calcium ions. The system was used to analyze compound-induced cell toxicity for several drugs that have been withdrawn from the market. The results show that this type of system helps to assess potential side effects of drug candidates. In addition, it can be used at different stages of the drug development process (see Fig. 2).
The application note Multiplexing calcium flux and metabolic effect measurements in iPSC cardiomyocytes describes the use of cardiomyocytes for the successful multiplexing of calcium flux and cell metabolism measurements in a microplate reader. Rhodamine 4 was used to detect calcium ions using fluorescence measurements on a CLARIOstar equipped with an Atmospheric Control Unit (ACU). The CLARIOstar and ACU not only enable incubation and analysis of the living cells in the same instrument but also permit detection of two assays in a multiplex approach. The use of cardiomyocytes represents a physiologically relevant platform that can be used for screening on and off target effects of drug candidates.
Induced pluripotent stem cells deliver many benefits to research efforts targeting atherosclerotic vascular disease. These flexible cells can readily differentiate into various cell types and can recapitulate disease-related phenotypes. They can therefore be used at different stages of the developmental or disease process to assess cell viability and cytotoxicity using assays like MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide), XTT (2,3-bis- (2-methoxy-4-nitro-5-sulphenyl)- (2H)-tetrazolium-5-carboxanilide, resazurin or CellTiter-Glo.
Induced pluripotent stem cells can also be genetically engineered to express reporter genes that indicate specific cellular responses. Reporter genes can be quantified by measuring fluorescence or luminescence signals, for example, in response to different conditions including drug treatments. This allows for screening of compounds that modulate specific molecular pathways or targets.
 Ion channel function is another area of interest where induced pluripotent stem cells can be used to good effect. Here microplate readers can be used to measure changes in membrane potential or ion flux using voltage-sensitive dyes or ion-selective fluorescent probes. This type of approach is particularly relevant but not limited to the study of arrhythmias.
Ion channel function is another area of interest where induced pluripotent stem cells can be used to good effect. Here microplate readers can be used to measure changes in membrane potential or ion flux using voltage-sensitive dyes or ion-selective fluorescent probes. This type of approach is particularly relevant but not limited to the study of arrhythmias.
Metabolic assays are also frequently used in the context of drug development. Researchers can assess parameters like oxygen consumption rate (OCR) or extracellular acidification rate (ECR) to study drug effects on cellular metabolism. Collectively, these types of measurements can provide insight on the overall metabolic function of cells, tissues and even organs and the impact of potential drugs on metabolism.
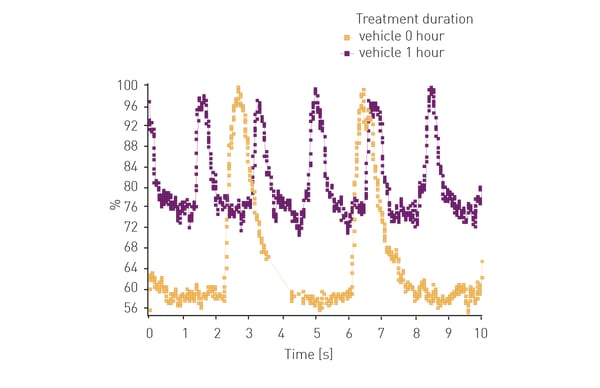
Another example is shown in the following application note: Lipid peroxidation assay in an ischemia-reperfusion cell model with NCLX inhibitors using the CLARIOstar® Plus. Ischemia reperfusion events lead to an increase in lipid peroxidation events in cells. In this example the CLARIOstar Plus with ACU was used to simulate ischemia (1% O2) and reperfusion (18% O2) to study potential inhibitors of lipid peroxidation in neuroblastoma cells using a fluorescent probe.
By mimicking the in vivo environment of atherosclerotic vascular disease scientists in basic research laboratories or drug discovery environments can make good use of microplate readers to advance their research.
BMG LABTECH microplate readers are ideally suited for the study of atherosclerotic vascular disease and the research for novel treatment strategies. BMG LABTECH´s range of modular multimode readers offers different options for this purpose.
The PHERAstar FSX was specifically conceived for screening campaigns and is your go-to reader for high-performance high-throughput screening with the fastest read times and compatibility with up to 3456-well plates.
Both the VANTAstar® and CLARIOstar Plus allow for wavelength flexibility and include Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options. Both readers can be equipped with an Atmospheric Control Unit to control CO2 and O2 in the reader, ideal for performing long-time cell-based studies.
Furthermore, the CLARIOstar Plus with ACU is the only reader on the market that can perform gas ramping. In addition to sustaining hypoxic and ischemic conditions, rapidly decreasing oxygen up to 0.1%, the ACU can also rapidly re-oxygenate the measurement chamber of the CLARIOstar Plus making it the only microplate reader on the market able to effectively simulate ischemic reperfusion.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities. In addition, the Omega series, CLARIOstar Plus and PHERAstar FSX microplate readers come with on-board injectors that can offer the very best options for detection at the time of injection.
Collectively, these multi-mode readers combine high performance with miniaturized assays, short measurement times, and offer considerable savings on materials and other resources.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
This article talks about ischemia reperfusion research and clarifies how you can mimic physiological and pathological gas conditions with the ACU accessory at the CLARIOstar plate reader.