Introduction
The human opioid system comprises four opioid receptors: µOR, δOR, κOR and NOPR (nociceptin opioid peptide receptor). Each of these G protein-coupled receptors has a set of related endogenous opioid peptides with distinct selectivity for their respective receptor. Structures of the entire human opioid receptor family with bound opioid peptides have been determined by cryo-electron microscopy and are now publicly available.1 Structure-guided biochemical studies have also been performed for each of these receptors and their mutants to better understand the mechanisms of how endogenous or exogenous peptides interact with the different types of receptors.
Currently opioid drugs are the strongest pain relievers available, but they are often accompanied by severe side effects. Researchers therefore want to better understand the mechanisms of peptide-receptor selectivity and drug signaling. By combining structural information with details on the mechanism of action, a framework is provided that facilitates the rational design of safer opioid drugs for pain relief.
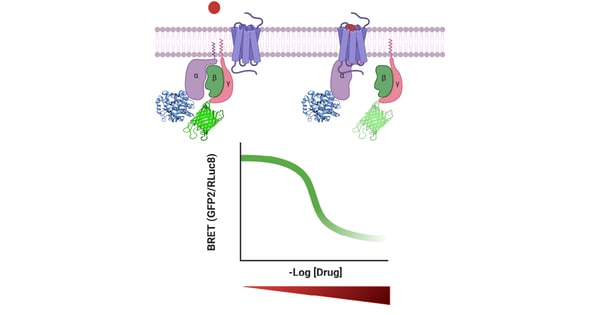
Here, to contribute to this framework, the PHERAstar FSX microplate reader was used for two types of biochemical assays: (1) G protein dissociation studies and (2) β-arrestin recruitment. β-arrestins turn off further signaling by the active receptor. They can also act as scaffolds for effectors mediating G protein-independent signaling. Gi dissociation concentration-response curves were obtained for peptide ligands at µOR, δOR, κOR and NOPR. In addition, both Gi dissociation and β-arrestin recruitment curves were obtained for mutants of each receptor.
Materials & Methods
- TRUPATH platform2
- PHERAstar FSX microplate reader (BMG LABTECH)
Experimental Procedure
- BRET assays were performed to measure G protein dissociation and β-arrestin recruitment at each of the different opioid receptors
- For G protein dissociation measurements, wild-type (WT) or mutant receptors were cotransfected with Gαi1-RLuc8, Gβ3 and GFP2-Gγ9 in a 1:1:1:1 ratio. Experiments were performed as previously described.2,3 Plates were read using a BMG LABTECH PHERAstar FSX with a BRET2 optic module
- For β-arrestin recruitment measurements, RLuc8 was cloned directly into the end of the C-terminus of WT and mutant µOR, δOR, κOR and NOPR, and experiments were performed as previously described.4,5 Plates were read with a PHERAstar FSX with a BRET1 optic module
Instrument settings
|
Optic settings
|
Luminescence, plate mode kinetic, simultaneous dual emission
|
|
| BRET1 |
Em: 475-30 |
|
|
BRET2
|
Em:410-80 Em: 515-30 |
|
|
Scan settings
|
Spiral average | |
| Scan diameter |
3 mm
|
|
|
General settings
|
Cycle time |
300 sec
|
| Measurement Interval time |
0.52 s per well
|
|
Results & Discussion
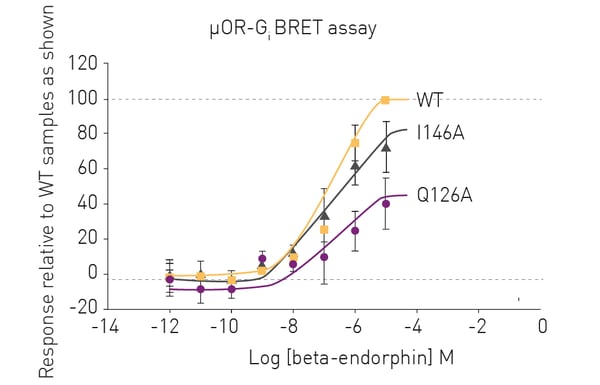
BRET assays provided quick and robust G protein dissociation measurements for the different opioid receptors in the presence of varying concentrations of their respective opioid peptides. The results in figure 2 show representative data for the BRET measurements of WT and mutant human µORs in the presence of β-endorphin. The BRET assays revealed distinct differences in the Gi protein dissociation measurements for the different mutants which in turn revealed the impact of the various mutations on the receptor. These findings serve as a useful starting point for future experiments looking at the mechanism of G protein signaling (figure 2).
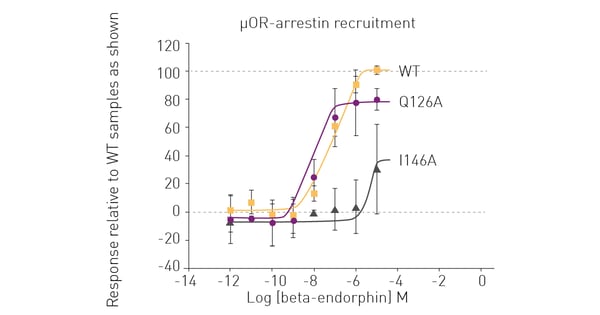
 BRET assays on the PHERAstar FSX also provided a reliable way to measure β-arrestin recruitment for the different opioid receptors in the presence of varying concentrations of their respective opioid peptides. Figure 3 shows representative data for the BRET measurements of WT and mutant human µOR in the presence of β-endorphin. The BRET measurements revealed distinct differences in the β-arrestin recruitment for the different mutants. This not only reveals the impact of each mutation on desensitization of the receptor signal but also suggests directions for future experiments. For example, mutants of κOR where β-arrestin recruitment is abolished may serve as a powerful starting point for the design of more potent G protein-biased agonists with fewer side effects.
BRET assays on the PHERAstar FSX also provided a reliable way to measure β-arrestin recruitment for the different opioid receptors in the presence of varying concentrations of their respective opioid peptides. Figure 3 shows representative data for the BRET measurements of WT and mutant human µOR in the presence of β-endorphin. The BRET measurements revealed distinct differences in the β-arrestin recruitment for the different mutants. This not only reveals the impact of each mutation on desensitization of the receptor signal but also suggests directions for future experiments. For example, mutants of κOR where β-arrestin recruitment is abolished may serve as a powerful starting point for the design of more potent G protein-biased agonists with fewer side effects.
Conclusion
The TRUPATH platform provided a robust and complete way to measure G protein signaling and β-arrestin recruitment for the human opioid receptor family using BRET1 and BRET2 assays. The different opioid receptors showed high selectivity towards Gi/o coupling rather than other G protein subtypes and the BRET assays provided an efficient and accurate way to monitor the human opioid receptor family. When used together with structures obtained from cryo-electron microscopy, TRUPATH BRET assays can serve to improve our understanding of the mechanism of peptide-receptor interactions and, in the longer term, facilitate a framework for the rational design of safer opioid drugs.
References
- Wang, Y., et al. Structures of the entire human opioid receptor family. Cell. (2023) 186: 413-427.
- Olsen, R.H.J., et al. TRUPATH, an open-source biosensor platform for interrogating the GPCR transducerome. Nat. Chem. Biol. (2020) 16: 841-849.
- DiBerto J.F., et al. Agonist and antagonist TRUPATH assays for G protein-coupled receptors. STAR Protoc.(2022) doi: 10.1016/j.xpro.2022.101259.
- Chakraborty S., et al. A Novel Mitragynine Analog with Low-Effi cacy Mu Opioid Receptor Agonism Displays Antinociception with Attenuated Adverse Effects. J Med Chem. (2021) 64(18):13873-13892. doi: 10.1021/acs. jmedchem.1c01273.
- Qu Q, et al. Insights into distinct signaling profi les of the µOR activated by diverse agonists. Nat Chem Biol. (2022) doi: 10.1038/s41589-022-01208-y.