Introduction
The moss Physcomitrella patens is a model organism in plant biology. A fast reproduction cycle, high rates of homologous recombination and the availability of efficient transformation methods of haploid protonema cells makes moss highly interesting as expression system for recombinant proteins. As a eukaryotic organism, the moss harbors all important post-translational modification features for production of biologically functional components, making it a superior choice over e.g. bacterial production systems.
In this application note we will show how to determine the total number of individualized viable moss cells per well by using the autofluorescence, represented by fluorescence from chlorophyll, and compare it with the relative number of freshly transformed, GFP-expressing cells. All fluorescence scans and intensity measurements have been performed with the CLARIOstar microplate reader from BMG LABTECH.
Materials & Methods
After generation of moss protoplasts from a bioreactor culture, the cells were transformed with a vector carrying the gene for GFP as fluorescent reporter, targeted to the nucleus. Non-transformed protoplasts were used as control. A serial dilution of these cells was done, leading to final theoretical concentrations of 225000 down to 110 cells per well. All dilutions were prepared in duplicates in black microplates with transparent bottom wells. A well filled with medium only was used as blank control. For autofluorescence and GFP measurements the CLARIOstar was set up with the following instrument protocol:
|
Detection mode: |
Fluorescence intensity |
|
Method: |
Bottom optic used |
|
Scan Mode: |
Orbital averaging |
|
Scan Diameter: |
3 mm |
|
No. of flashes per well: |
8 |
Optic settings for autofluorescence
LVF monochromator: 475-30/680-20
Optic settings for wtGFP measurements
LVF monochromator: 385-12/510-20
Results & Discussion
Determining the relative number of transformed moss cells using chlorophyll autofluorescence and GFP
Objective of the experiment was to determine the minimum necessary number of GFP-expressing cells for measurement in the fluorescence reader. In the future, relative expression rates of recombinant proteins in different moss strains could be comparable by using a defined number of moss cells during measurements. An easy way to calculate the total number of viable cells present in the microplate well is using the autofluorescence of moss cells. Moss cells contain chlorophyll a and b. The far red emission of these pigments can be used as a tool for cell counting.
Fig. 1 shows the emission scan of untransformed moss cells as well as moss cells expressing GFP.
A clear peak at around 684 nm can be found in both kinds of moss cells indicating chlorophyll autofluorescence. The emission of wild type GFP at around 510 nm can only be found for GFP expressing cells. From excitation and emission scans optimal LVF monochromator chlorophyll wavelengths have been determined: excitation 475-30 and emission 680-20.
From the measurements, a standard curve could be calculated showing the relation of the chlorophyll autofluorescence to the theoretical number of moss cells (Fig. 2).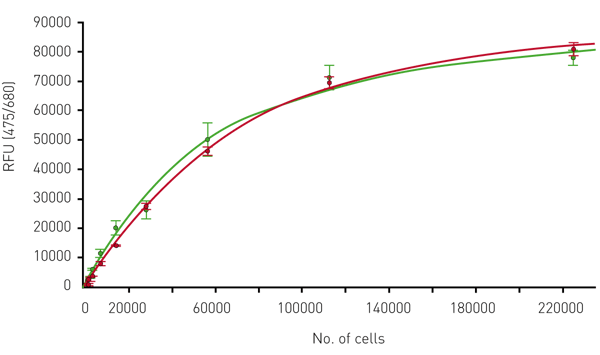
The two curves are very similar, indicating that the autofluorescence is a useful tool to determine the total number of viable cells. From the standard curves, we concluded that the detection limit via autofluorescence for moss cells is around 500 cells per well.
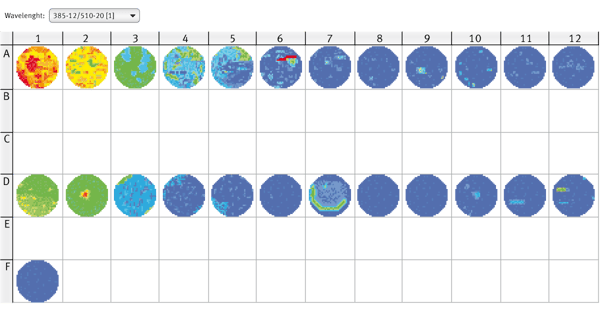
Well scan comparison of GFP expressing cells and mock control
The distribution of protoplasts in the well may not be even. To investigate this assumption, a well scan was carried out using samples with GFP-expressing moss cells, non-transformed cells and a medium-only control.
The results (Fig. 3) clearly demonstrate that the moss cells are indeed not distributed evenly in the wells. Some wells show aggregates, which would have negative influence on the reliability of results from a single measurement in the middle of the well. Therefore, it is recommended to use the orbital averaging function. In this mode, the measurement takes place on an orbit with definable diameter in the well. The average value of all orbit measurement points will be displayed. The use of the orbital average function will result in more stable values with decreased deviation of replicate wells.
In Fig. 3 it is shown that GFP expressing moss cells show higher fluorescence values compared to the mock control. However, also the control shows an increase in fluorescence with increasing number of cells. Thus, it can be concluded that chlorophyll is also excited by the excitation wavelength of 385 nm, and a non-transformed control is necessary for background subtraction. In this experiment, the transfection rate of GFP-construct into moss cells appeared to be around 20 %. Higher transfection rates would result in a better discrimination of GFP-expressing moss cells and the mock control and therefore in a lower total number of cells required for measurements.
Conclusion
Using the CLARIOstar microplate reader, GFP and chlorophyll emission spectra can be taken to optimize measurement settings to special medium or buffer conditions. The total number of moss cells can be determined by a standard curve using chlorophyll fluorescence emission. Moss cells down to 500 cells per well can be detected.
For analysis of GFP expressing moss cells, it is necessary to use non-transformed cells as background control, as some part of the measured fluorescence emission is related to chlorophyll.
About Greenovation
Greenovation develops plant-made next-generation therapeutics using its proprietary BryoTechnology platform. The company aims to optimize the production of highly-efficient glycoproteins for the treatment of rare diseases.
*In 2020, the company Greenovation Biotech GmbH was transformed into a new company, Eleva GmbH.