
PHERAstar FSX
Powerful and most sensitive HTS plate reader
Tryptophan fluorescence can be used to study protein conformation. Find out how microplate readers can be used to detect tryptophan and investigate protein function.
 Dr Barry Whyte
Dr Barry Whyte
Barry Whyte is Application Scientist and Science Writer at BMG LABTECH in the United States. He has PhD and Bachelor of Science (BSc) degrees in biochemistry from the University of Bristol in the United Kingdom and more than 20 years of experience in the life sciences and science communications. Over the years, Barry has worked on three continents and traveled widely. He enjoys building on his international work experience and learning new ways to help scientists advance their research.
Proteins are indispensable molecular catalysts of biological reactions and play important structural roles in cells. The way they fold or unfold reveals crucial information about their biological activity.
The fluorescence of naturally occurring amino acids like tryptophan is a useful tool to study protein structure and function. Intrinsic tryptophan fluorescence can be used for example to look at conformational changes, measure enzyme activities, or investigate the binding of ligands. It can also provide insight on the impact of mutations or other protein modifications on protein stability or protein aggregation.
Proteins are built from 20 or more naturally occurring amino acids. Three of these amino acids — tryptophan, tyrosine, and phenylalanine — have ring structures with fluorescent properties. Of the three, tryptophan exhibits the most suitable properties for detection since it contributes most to the intrinsic fluorescence emission of most proteins.
Tryptophan is a relatively rare amino acid. Typically, proteins contain either one or a few tryptophan residues which translates into sensitive fluorescence measurements for studies of conformational changes.
Its aromatic residue means tryptophan can also be measured by absorbance. But fluorescence gives researchers even more sensitive detection of tryptophan.
Significantly, the fluorescence emission of tryptophan residues is higher when this amino acid is on the outside of a protein. This makes fluorescence emission a useful indicator of the conformational state of a protein and its folding process. If tryptophan finds itself in a more interior environment of a protein’s 3D structure, its fluorescence emission decreases.
The application note “Tryptophan quantification using UV fluorescence measurements on the CLARIOstar® multi-mode microplate reader” describes how tryptophan fluorescence can be detected at high sensitivity and accuracy on BMG LABTECH microplate readers. Here, a combination of filters for excitation in the UV range and the Linear Variable Filter (LVF) monochromator™ for emission on the CLARIOstar Plus were used to achieve excellent sensitivity with a limit of detection (LOD) of less than 2 nM tryptophan (fig. 1) which corresponds to a concentration of 0.4 ng/ml.
GTP-binding proteins or G proteins are an important family of molecular switches in cells. They are involved in transmitting crucial cell signals from outside a cell to its interior. Two classes of G proteins are defined. The first functions as monomeric small GTPases (small G-proteins). The second class comprise heterotrimeric G-protein complexes. Heterotrimeric G-proteins consist of three subunits (Gα, Gβ, and Gγ) that are typically bound to a seven-pass-transmembrane domain receptor, also known as G-protein coupled receptor (GPCR). G-protein coupled receptors and G proteins work together to transmit signals from many hormones, neurotransmitters and other signaling factors. These signals lead to a cascade of interactions and enzyme activities in the signaling machinery which ultimately lead to a change of cell function. Due to their role in cellular metabolism and disease, GPCRs are the largest family of targets for approved drugs. As these processes are not completely understood, scientists are interested in working out what happens to G-proteins when the cascade of interactions is triggered.
The application note “Using intrinsic tryptophan fluorescence to measure heterotrimeric G-protein activation” describes an automated assay system for measuring G-protein α subunit activity on a microplate reader using fluorescence, an approach that could be used for the screening of G-protein inhibitors. In this study the activation of the G-protein α-subunit was measured by looking at the fluorescence of a highly conserved tryptophan residue located in the switch II region of the G-protein α-subunits (fig. 2). The conformational change in the switch region results in an increase in tryptophan emission fluorescence at 350 nm upon excitation at 280 nm. This is a good example of how BMG LABTECH plate readers can be used to look at conformational changes using fluorescence.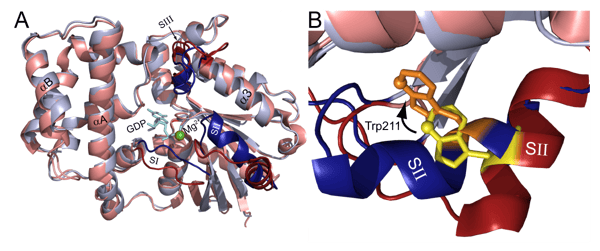
Conformational changes within proteins and the corresponding changes in fluorescence emission can also be used to look at protein-ligand interactions. For example, intrinsic tryptophan fluorescence can be used to measure the binding of an antibody to its antigen, as conformational changes within the binding site or simply the presence of ligand can result in changes in tryptophan fluorescence.
In the application note “Investigation of the stereoselectivity of an anti-amino acid antibody utilizing tryptophan fluorescence”, BMG LABTECH microplate readers were used to monitor antibody binding by measuring tryptophan fluorescence with high sensitivity and good signal-to-noise ratios. This study looked at the binding of a stereoselective antibody specific for the D-enantiomer of phenylalanine and other amino acids. Higher concentrations of the antibody resulted in increasing fluorescence emission at 350 nm upon excitation at 280 nm. No increases in fluorescence were observed with the L-enantiomers of the corresponding amino acid. In addition to phenylalanine, similar results were obtained with the enantiomers of five other amino acids. The effects of analyte concentration, temperature and pH were investigated to establish optimal conditions for the binding reaction. Tryptophan fluorescence can therefore be employed to investigate protein-ligand interactions for different applications including the deduction of binding affinities.
Tryptophan fluorescence can also be a useful tool for the investigation of protein unfolding. While Φ values, a numerical indicator obtained from protein engineering experiments, are often used to look at the folding transition states of small proteins1, in most cases these methods do not adapt well to the needs of high throughput screening. For this purpose, tryptophan fluorescence is an effective solution to directly measure the unfolding rates of proteins which can also be employed as a useful indicator of protein stability.
In the application note “High-throughput kinetic protein unfolding assays developed on a BMG LABTECH Omega series microplate reader” detection of tryptophan fluorescence was used to look at two types of protein unfolding assays. A kinetic unfolding assay involved injecting a denaturant into a well on a microplate and monitoring the fluorescence emission of the intrinsic tryptophan residues. In the second approach, a limited proteolysis assay was used for proteins with larger kinetic constants of unfolding. Reading at the time of injection and high frequency measurements every 120 ms provided maximal signal retention. Unfolding constants could be calculated from the data using Michaelis-Menten equations. Both methods provided reliable kinetic protein unfolding measurements at high throughput. The reactions could be performed at protein concentrations that conserve samples.
What is the preferred BMG LABTECH microplate reader for specific needs and applications for protein folding and conformational state?
The Omega series of microplate readers provide an ideal platform for often used applications in the life science laboratory. They are well suited for the detection of tryptophan fluorescence in studies of protein-ligand interactions or to deduce binding affinities. All BMG LABTECH microplate readers have exceptionally fast reading capabilities. Like other BMG LABTECH microplate readers, the Omega series and PHERAstar® FSX offer options for inclusion of on-board injectors that provide the very best solutions for detection at the time of injection.
Both the VANTAstar® and CLARIOstar® Plus allow for fluorescence emission scanning and include the Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of the highly flexible Linear Variable Filter monochromators and different filter options. The outstanding performance levels of both microplate readers are suitable for more advanced studies of protein-ligand interactions, enzymatic reactions, assay development, and fast kinetic studies using tyrosine fluorescence. They are highly effective solutions to investigate protein folding or conformational state.
The PHERAstar FSX is the option of choice if researchers need the highest possible sensitivity and the fastest speed at scale for their protein folding or conformational state measurements. The PHERAstar FSX was specifically conceived for screening campaigns and offers the level of performance suitable for high-throughput investigations.
Collectively, these multi-mode readers combine high-quality measurements with miniaturised assays, short measurement times, and offer considerable savings on materials and other resources.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Binding constants quantify the strength of a binding reaction between a biomolecule and its target (ligand). But how do you measure them and what can you do with them?
Receptor-ligand kinetics is the study of the rates at which receptors and ligands interact, bind and dissociate. Learn why these types of measurements are important and how to measure them.
Receptor-ligand interactions are crucial for cell signalling. They are also important for drug discovery. How do microplate readers deliver benefits to both?
Degrons are specific sequences of amino acids or structural motifs within a protein that are important for targeted protein degradation. Find out how microplate readers can advance research into natural and engineered degrons.
Nitration can significantly modify the structure and function of proteins in cells. But how can measurements of protein nitration reveal crucial information about biological activity and how can microplate readers help?
Innovation for targeted protein degradation and next-generation degraders is gathering pace. This blog introduces some of the different approaches that act via the lysosome or proteasome.