
PHERAstar FSX
Powerful and most sensitive HTS plate reader
Second messengers play a pivotal role in signal transduction events in cells. But how can these small, transiently lived molecules be detected and how can microplate readers help?
 Dr Barry Whyte
Dr Barry Whyte
Second messengers are small molecules or ions that transmit signals received at the cell surface to the inside environment of the cell (Fig. 1). 1 These small, rapidly diffusing effectors are involved in a cascade of signal transmission events that regulate essential biological processes like cell growth, metabolism, and gene expression. In this blog, we look at the preferred ways to examine signal transmission events and how these different approaches can advance research quickly and effectively using microplate readers.
The world of second messengers comprises a wide variety of molecules and ions with properties that make them suitable for the task of intracellular messaging. Second messengers are united by their ability to bind to specific protein targets to alter their activity and elicit downstream signals within the cell. Some of the most well-known examples include cAMP, calcium ions, inositol trisphosphate, and diacylglycerol (Fig.2). For the most part, second messengers can be grouped into three major classes: cyclic nucleotides, lipids, and ions. In addition, other molecules like gases or free radicals have been shown to serve as signal transmission agents.
cAMP and cyclic guanosine monophosphate (cGMP) are the most well-characterized cyclic nucleotides serving as second messenger molecules in the cell. Like other second messengers their levels are tightly controlled in time and space, and they can travel and diffuse rapidly within the cell for signal transmission.
The concept of cAMP as a second messenger arose through the groundbreaking work of Earl Sutherland and colleagues. In 1958, Sutherland authored two papers that introduced the idea of cAMP as a second messenger or intermediary in hormone signalling.2,3 This work laid the foundation for understanding the role of cAMP in intracellular signal transmission and its involvement in different cellular processes. Today cAMP is known to be involved in many cellular processes and signal transmission events which include the regulation of enzyme activities, ion channels, and gene transcription.
cAMP is one of the critical downstream effectors of G protein-coupled receptors for signal transmission. GPCRs are the largest and most diverse group of membrane receptors in eukaryotes and serve as a gateway to many cellular responses. Their distribution and involvement in many crucial signal transmission pathways make them popular targets for drug discovery and development. You can read more about the way GPCRs function in the BMG LABTECH blog G protein-coupled receptors (GPCRs).
Inside the cell, one of the main roles of cAMP is to activate the enzyme protein kinase A (PKA), a serine-threonine kinase (Fig.3). Phosphorylation is one of the main mechanisms to regulate protein function in response to extracellular stimuli. In the case of PKA, cAMP binds to the regulatory subunit of the enzyme leading to activation of its catalytic subunits. In a typical cell, 300-500 enzymes are estimated to be phosphorylated by PKA which reflects the fine control cAMP exerts on the intracellular environment.1 In addition, the activated form of this enzyme can commute to the nucleus where it can directly influence transcriptional activation and reprogramming of the cell.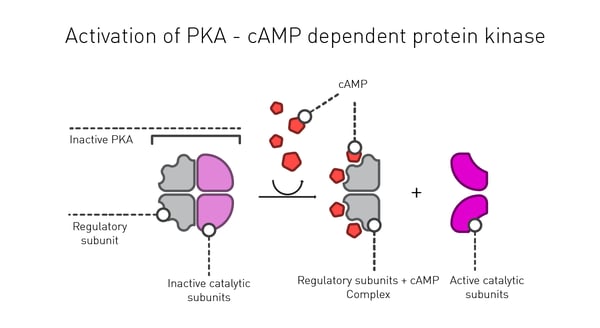
3’-5’-Cyclic guanosine monophosphate (cGMP) is a versatile second messenger that is involved in different physiological processes and signal transmission events. For example, cGMP plays roles in vasodilation, phototransduction and neurotransmission where it is known to function in intracellular signal transmission. In the case of a guanylate cyclase-linked receptor, the binding of an effector activates guanylate cyclase which subsequently converts guanosine triphosphate (GTP) into cGMP.
cGMP activates specific target proteins or enzymes that include cyclic nucleotide-gated ion channels, cAMP-dependent protein kinases such as protein kinase G (PKG), and phosphodiesterases. PKG is one of the key effector proteins activated by cGMP and can result in changes in ion channel activity, gene expression and the relaxation of smooth muscle. The signaling of cGMP is terminated by the action of phosphodiesterase enzymes that convert cGMP into inactive GMP.
Diacylglycerol (DAG) and phosphatidylinositol 4,5-bisphosphate (PIP2) are both lipid molecules. PIP2 is strictly speaking not a second messenger but is the precursor for both DAG and inositol-1,4,5-trisphosphate (IP3), both of which are bona fide second messengers involved in signal transmission. IP3 and DAG are both produced when GPCRs are activated.
DAG is a biologically active lipid that resides in the cell membrane where it exerts its actions.
One of the main roles of DAG is to activate protein kinase C (PKC). Activated PKC phosphorylates a wide range of target proteins within the cell which leads to signal transmission and regulation of different cellular processes including cell growth, ion channel function, secretion, phagocytosis, neurotransmission, metabolism, and gene expression.
IP3 plays a key role in controlling calcium levels in the cell which in turn regulates various cellular processes (see next section). After phospholipase C liberates IP3 by breaking down PIP2, IP3 is released into the cytoplasm where it binds to IP3 receptors on the endoplasmic reticulum (ER). This binding causes the release of calcium (Ca2+) from the ER which activates subsequent processes in the cell including those regulated by proteins like Ca2+-activated calmodulin. Calmodulin mediates many processes including inflammation, metabolism, cell death, immune responses, and memory.
Ions like Ca2+, potassium (K+) and magnesium (Mg2+) play essential roles in the function of cells. These include activities where they function as cofactors for enzymes and structural proteins, participate in the transmission of nerve impulses, and help control muscle contraction by interaction with regulatory proteins.
Ca2+ also plays a role as a second messenger in cells by regulating certain protein targets during signal transmission. As such it has many physiological duties in signal transmission including muscle contraction, neuronal transmission, cell motility and cell growth to name a few.
As mentioned in the previous section, one of the key effectors Ca2+ regulates is the protein calmodulin (Fig.4). When calcium concentrations increase, calcium ions bind to calmodulin. This can lead to calmodulin associating with some targets or being displaced from others. In the case of phosphorylase kinase, calmodulin is a subunit of the enzyme that binds calcium and activates the enzyme when calcium ion concentrations are high. Indeed, calmodulin often serves as a calcium-sensitive switch for the proteins it regulates, and this is a general theme for the way it functions in signal transmission events. The role of Ca2+ in signal transmission and tools to measure Ca2+ are discussed in more detail in the blog Calcium assays: at the centre of biology.
Gases or free radicals also serve as signal transmission agents. Nitric oxide (NO) 4 is a gas that can serve as a second messenger in signal transmission by activating guanylate cyclase. This leads to the production of cGMP and activation of the cellular processes outlined earlier. NO is synthesized by nitric oxide synthase (NOS) enzymes that convert the amino acid L-arginine into NO and L-citrulline. Once it is made in specific cells, NO is released into the extracellular space where it can diffuse across cell membranes and enter target cells to function as a signaling molecule. The cascade of changes arising from NO produced by different NOS enzymes influences a wide range of processes including muscle relaxation, the regulation of blood pressure, and neurotransmission.
Other emerging areas of second messenger signal transmission in cells include the actions of reactive oxygen species (ROS). ROS are typically known for their ability to cause damage to cells through oxidation reactions which can in turn lead to disease. They act as second messengers in some cellular processes but in many cases the details of how they function in signal transmission remain to be established. While there is evidence that suggests that ROS can serve as signal transmission molecules in specific contexts, they are not as well established in the roles of traditional second messengers as effectors like cAMP, IP3 or Ca2+. Some of the areas where ROS molecules have been shown to serve as second messengers include in immune responses to kill invading pathogens and in mitochondria for processes like autophagy and apoptosis.
Applications involving second messengers and signal transmission find uses in many research areas ranging from cell and molecular biology to high throughput screening and drug discovery which makes them indispensable tools for life science research. When looking to quantify second messengers using microplate readers, fluorescence and luminescence-based methods are the preferred choice, because they usually provide higher sensitivity. Microplate readers offer precision and high sensitivity for the quantification of second messengers in cells. They also provide the high throughput needed for drug discovery applications related to signal transmission events.
The broad spectrum of solutions offered by microplate readers makes them indispensable tools to investigate the impact of second messengers. BMG LABTECH offers many options that cater to different assays for the measurement of second messengers (see Table 1 second messenger application notes). Here we outline some of the detection capabilities of microplate readers that can be used for second messenger detection and provide application examples for different detection modes.
BMG LABTECH Application Notes
The application note Dual channel kinetic assays for detecting ligand bias at GPCRs describes the use of red and green fluorescent biosensors to capture second messenger signaling responses over time. Biosensors for beta-arrestin, cAMP, DAG, Ca2+, and PIP2 have been developed and can be combined in a single assay to simultaneously measure response functions in the same population of cells. These fluorescent biosensors were monitored on a BMG LABTECH CLARIOstar with high precision.
In the application note Real-time detection of Gs and Gi signaling in living cells genetically encoded biosensors were used to measure the levels of cAMP, generated in response to GPCR activation, in a fluorescence assay on the CLARIOstar microplate reader. When cAMP binds to these different protein sensors, conformational changes result which lead to measurable changes in fluorescence. The sensors come in either a red or green version that can be spectrally resolved in the living cell. The systems were used to detect Gs and Gi signaling in HEK293 cells which allowed for the detection of GPCR signal transmission events and the measurement of increases in cAMP production.
Fluorescence Resonance Energy Transfer (FRET) measurements can also be used to measure cAMP levels. In the application note Cell-based assay detects residual beta-blocker substances in effluent of municipal wastewater treatment plants the CLARIOstar microplate reader was used to enable kinetic well scans of a FRET-based cAMP biosensor. The cAMP sensor CEPAC [cAMP detecting FRET biosensor mCerulean – Epac (exchange protein directly activated by cAMP) – mCitrine] displays FRET from the blue (mCerulean) to the yellow (mCitrine) fluorophore. When cAMP binds to the sensor, FRET is diminished and the changes in fluorescence intensity can be measured.
In the application note Simultaneous detection of GPCR second messengers in living cells a PIP2 sensor was used to determine PIP2 kinetics on a CLARIOstar simultaneously with the measurement of the kinetics of DAG formation. Multiplexing of both second messengers DAG and PIP2 was used to monitor signal transmission cascades using these kinetic readouts of fluorescence. It was also possible to multiplex DAG and Ca2+ kinetics.
In the case of IP3, several alternative technologies are available to measure this second messenger. One distinction in these options is whether IP3 is measured directly or indirectly. The short half-life of IP3 makes it unsuitable for direct measurement with most detection methods. The IP-One (inositol trisphosphate-1) assay from Cisbio circumvents this restriction by allowing measurement of the accumulation of IP1, a downstream molecule in the IP3 cascade, instead of the transient messenger IP3. The IP-One assay with homogeneous time-resolved fluorescence (HTRF) technology works by allowing for the sensitive and quantitative surrogate measurements of IP3 which is also suitable for high-throughput measurements.
The application note HTRF IP-One assay used for functional screening describes the use of TR-FRET for the high throughput screening of the MRCT 100K compound collection for the galananin receptor. The IP-One HTRF® assay from Cisbio was shown to be a fast and reliable method to directly screen many compounds using the PHERAstar microplate reader. A robust assay was established using fresh or Frozen Instant Cells.
In the application note GPCR activation is measured using Cisbio’s cAMP and IP1 HTRF® HTplex™ cell-based assay, IP1 levels were determined to investigate the activation of the vasopressin-2 receptor in Chinese Hamster Ovary (CHO) cells. The HTplex™ assay makes use of the Lumi4-Tb™ TR-FRET Cryptate chemistry which allows for detection of two signal transmission events in one well using two different acceptors. This assay can therefore also be used to measure cAMP levels in parallel to IP3 which means it can evaluate two different GPCR signal transmission pathways simultaneously.
DiscoverX has developed a homogeneous assay based on fluorescence polarization to directly measure IP3 levels generated by GPCR activation. In the application note HitHunter® IP3 assay for GPCR screening using the PHERAstar® FS, the HitHunter® assay was shown to be a robust and sensitive assay for the detection of basal levels of IP3 in cell lysates as well as the low levels of IP3 required for induction. The assay is based on fluorescence polarization using a proprietary binding protein that is highly selective for the active isomer of IP3. The assay is a competitive binding assay in which cellular IP3 displaces a fluorescent derivative of IP3 from a specific binding protein. One of the features that sets this assay apart is its sensitivity and its ability for miniaturization in microplates up to 1536-well formats.
The application note Monitoring intracellular Ca2+ fluxes and cAMP with primary sensors from Lonza describes different sensors that can be used to measure second messengers. cAMP was measured using luminescence detection of the GloSensorTM cAMP assay from Promega. In this system, firefly luciferase is fused genetically to the cAMP-binding domain of human protein kinase A. When cAMP binds to the cAMP-binding domain, the whole protein undergoes a conformational change that activates the luciferin domain. Luciferin is converted to oxyluciferin which results in an increase in luminescence.
cAMP assays were performed using human mesenchymal stem cells treated with forskolin on the Omega series, CLARIOstar Plus and PHERAstar FSX microplate readers. In the same application note, the use of a biosensor to measure calcium is described. The incubation of human umbilical vein endothelial cells (HUVEC) expressing the i-Photina® apophotoprotein with coelenterazine in the presence of oxygen leads to the formation of a stable complex: the active photoprotein. Calcium released from intracellular stores upon stimulation of the cells with agonists via G-protein coupled receptors binds to the photoprotein. The excited photoprotein converts coelenterazine to coelenteramide and emits a flash of blue luminescence.
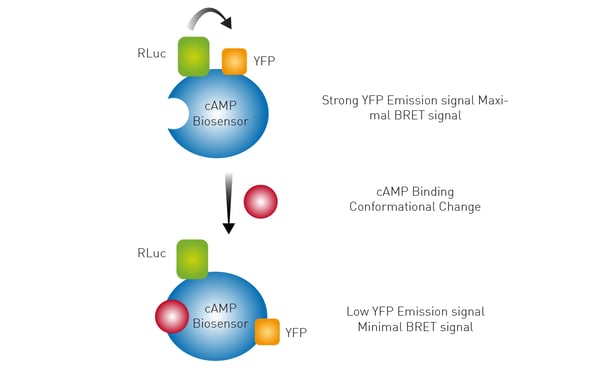
The use of a Bioluminescence Resonance Energy Transfer (BRET) biosensor is outlined in the application note Monitoring receptor ligand binding in living cells. Here a conformational change induced by cAMP binding to the biosensor results in an increase in distance between the Renilla luciferase (RLuc) and yellow fluorescent protein (YFP). This change results in a lower BRET signal. Forskolin-treated samples showed a decrease in BRET ratio and the CLARIOstar microplate reader provided the sensitivity needed to detect the changes in luminescence.
The application note HitHunter® cAMP XS+ assay for GPCR screening using the PHERAstar FSX describes a luminescence-based detection method that measures changes in intracellular concentrations of cAMP. The HitHunter® cAMP XS+ assay from DiscoverX is based on the complementation of two fragments of Escherichia coli beta-galactosidase and competition for binding to an antibody specific for cAMP. Free cAMP generated by the cell can competitively displace enzyme donor-conjugated cAMP from the antibody. This allows the enzyme donor-conjugate to freely complement inactive enzyme and thus generate a luminescence signal directly proportional to the amount of cAMP present in the cell. The HitHunter® cAMP XS+ assay from DiscoverX is particularly useful for screening challenging targets with low basal levels of cAMP or low levels of cAMP response. Changes in the intracellular cAMP levels correlate with GPCR activation and measurements are suitable for high-throughput screening.
Enzyme-linked immunoabsorbent assays (ELISAs) are also available to measure the levels of cAMP, cGMP or other components of signaling pathways and researchers may choose from different commercially available kits that allow for their detection on microplates. Detection may be achieved by measuring absorbance or fluorescence. Furthermore, the availability of incubation and gas control on BMG LABTECH readers equipped with Atmospheric Control Unit (ACU) allows the performance of complex cell-based assays that permit second messenger measurements.
BMG LABTECH microplate readers are ideally suited for the study of second messengers and signal transmission. Cell signaling events rarely occur alone and take place simultaneously or in concert. BMG LABTECH´s range of modular multi-mode readers offers different options for this purpose and offer the possibility of reading several assays in just one well. The simplicity of combining different fluorophores in a kinetic measurement with BMG LABTECH instruments qualifies them to read real-time multiplex signal transmission assays.
The PHERAstar FSX was specifically conceived for screening campaigns and is your go-to reader for high-performance high-throughput screening with the fastest read times and compatibility with microplates up to 3456-well formats.
Both the VANTAstar® and CLARIOstar Plus allow for wavelength flexibility and include Enhanced Dynamic Range technology for superior performance in a single run. They also offer increased light transmission and sensitivity courtesy of Linear Variable Filter MonochromatorsTM and different filter options. Both readers can be equipped with an Atmospheric Control Unit to control CO2 and O2 in the reader, ideal for performing lengthy cell-based studies. Furthermore, the CLARIOstar Plus with ACU is the only reader on the market that can perform gas ramping. Additionally, the CLARIOstar Plus allows measurement of 100 data points per second. This high sampling rate is very important in fast kinetic reactions, allowing for example calcium transient measurements in real time.
All BMG LABTECH microplate readers have exceptionally fast reading capabilities. In addition, the Omega series, CLARIOstar Plus and PHERAstar FSX microplate readers come with on-board injectors that can offer the very best options for detection at the time of injection. The VANTAstar® can be equipped with an injection module which further includes a software-controlled heater and stirrer to support the preparation and temperature adjustment of reagents.
Collectively, these multi-mode readers combine high performance with miniaturized assays, short measurement times, and offer considerable savings on materials and other resources.
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
Life in the depths of the ocean operates under extreme conditions. Find out how proteins from deep-sea luminescent organisms are useful for measurements on microplate readers.
NanoBRET is used to analyse binding events, signaling pathways and receptor trafficking in live cells and has significantly expanded the range and applications of BRET assays.
Find out about the different types of cell-based assays and why they have become an indispensable tool in a such a broad variety of disciplines in this blog article.
Read here about the various threats associated with mycoplasma contamination and find out about methods to prevent, detect, and eliminate mycoplasma in your cell culture.
Apoptosis is a form of programmed cell death responsible for the removal of damaged or unnecessary cells. Read more about available apoptosis assays here.