Introduction
Bioluminescence Resonance Energy Transfer (BRET) is a system of choice for monitoring intermolecular interactions in vivo. BRET is an advanced, non-destructive, cell-based assay technology that is perfectly suited for proteomics applications, including receptor research and the mapping of signal transduction pathways. The assay is based on non-radiative energy transfer between fusion proteins containing Renilla luciferase (Rluc) and e.g. Yellow Fluorescent Protein (YFP). The BRET signal is generated by the oxidation of a coelenterazine derivative substrate.
For this application note, the BRET2™ demo kit has been used to prove the feasibility of performing a BRET assay on a BMG LABTECH microplate reader. The BRET demo kit applies the cell-permeable and non-toxic coelenterazine derivative substrate DeepBlueC™ (DBC) and a mutant of the Green Fluorescent Protein (GFP2) as acceptor. These compounds show improved spectral resolution and sensitivity over earlier variants.
Materials & Methods
All materials were obtained through normal distribution channels from the manufacturers stated.
- BRET2™ demo kit, PE Life Sciences
- White 384-well OptiPlate™, PE Life Sciences
Experimentals
A description for the development of BRET2™ protein-protein interaction assays is included with the demo kit. The following section focuses on the microplate reader settings recommended in the assay protocol.
BRET2TM demo kit reagents:
- Non-transfected CHO cell extracts
- Negative control (Rluc + GFP2 not fused together)
- Positive control (Rluc-GFP2 fused together)
- DeepBlueC™
- BRET2™ assay buffer
Assay Protocol (for a white 384-well plate)
1. Addition of BRET2™ assay buffer:
- A10-D12: 15 µL of BRET2™ buffer
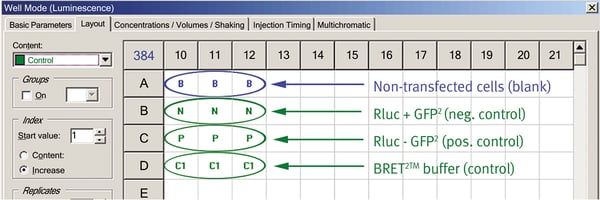
2. Addition of 10 µL of each cell extract (Fig.1):
- A10-A12: Non-transfected cells (blank)
- B10-B12: Neg. BRET2™ control (Rluc + GFP2)
- C10-C12: Pos. BRET2™ control (Rluc-GFP2)
- D10-D12: BRET2™ assay buffer
3. Automated injection of DBC and measurement:
Insert the prepared plate in the instrument and fill the injector with DBC solution.
- A10-D12: Injection of 25 µL of DBC at 10 µM
Instrument settings
|
FLUOstar Omega
|
CLARIOstar
|
PHERAstar
|
|
|
Detection Mode
|
Luminescence, Well Mode
|
||
|
Simultaneous dual emission
|
√
|
||
|
Sequential dual emission
|
√
|
√
|
√
|
|
Filter settings
|
410-80
515-30
|
410-80
515-30
|
BRET2 optic module
|
|
Gain
|
3800
|
3800
|
3800
|
|
Measurement Interval time (seconds)
|
1.0
|
1.0
|
0.02
|
|
No. of. Intervals
|
50
|
50
|
50
|
|
Injection start time (seconds)
|
0
|
0
|
0
|
|
Pump speed (µl/s)
|
260
|
260
|
260
|
Results & Discussion
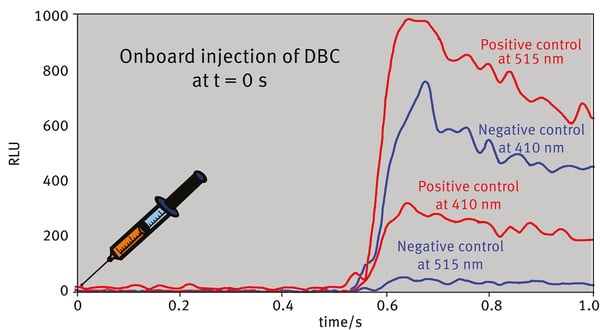
When the donor and acceptor are in close proximity, the energy resulting from the catalytic degradation of the DBC is transferred from Rluc to GFP2 which will then emit fluorescence at its characteristic wavelength.
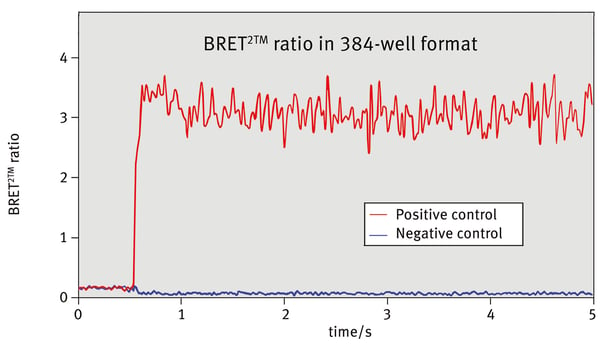
The kinetic curves (raw data - blank) of the negative control are shown in Fig.2 for both channels. The low values of the 515 nm channel indicate that no resonance energy transfer occurred. Whereas the positive control shows reduced values at the 410 nm and elevated values at the 515 nm channel due to the BRET effect.
 The calculated BRET ratio indicates the occurrence of protein-protein interaction in vivo. This type of detection eliminates data variability caused by fluctuations in light output which can be found with variations e.g. in assay volume, cell types, number of cells per well, and/or signal decay across the plate.
The calculated BRET ratio indicates the occurrence of protein-protein interaction in vivo. This type of detection eliminates data variability caused by fluctuations in light output which can be found with variations e.g. in assay volume, cell types, number of cells per well, and/or signal decay across the plate.
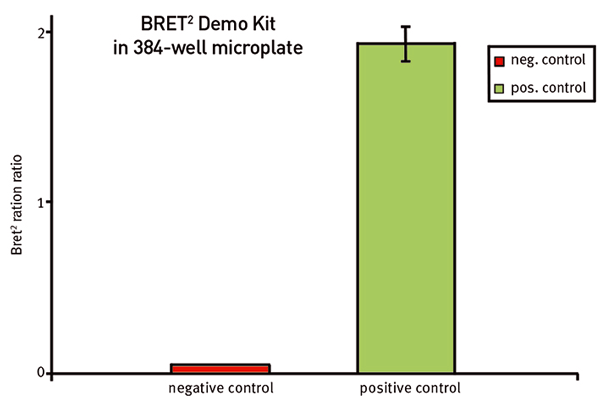
In Fig. 3 the blank corrected BRET2™ ratios for both, negative and positive control, are shown and were determined as:
 The MARS Data analysis software offers easy-to-use tools to do the ratio calculation in just 1 mouse click. The signal for negative and positive control for measurements using simultaneous dual emission reveals a value of around 0.06 and 3.3 respectively, which leads to a factor of around 50 and clear discrimination between these controls. The signal for negative and positive control for measurements using sequential dual emission lead to values of around 0.05 and 1.9 respectively, which leads to a factor of around 40 (Fig. 4).
The MARS Data analysis software offers easy-to-use tools to do the ratio calculation in just 1 mouse click. The signal for negative and positive control for measurements using simultaneous dual emission reveals a value of around 0.06 and 3.3 respectively, which leads to a factor of around 50 and clear discrimination between these controls. The signal for negative and positive control for measurements using sequential dual emission lead to values of around 0.05 and 1.9 respectively, which leads to a factor of around 40 (Fig. 4).
The high factor between these controls is caused by the artificial fusion construct of the positive control (Rluc-GFP2) resulting in an extremely high BRET. Real assay samples will presumably result in lower ratios. Nevertheless, BRET assays show no photo-bleaching or photo-isomerization of the donor protein or auto-fluorescence from cells or microplates. Furthermore, the large spectral resolution between donor and emission peaks in BRET2TM (115 nm) greatly improves the signal to background ratio over traditionally used BRET and FRET technologies that typically have only a 50 nm spectral resolution.