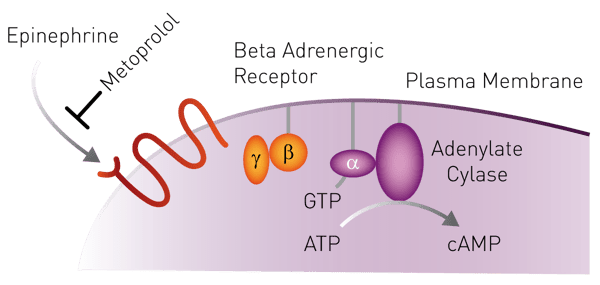
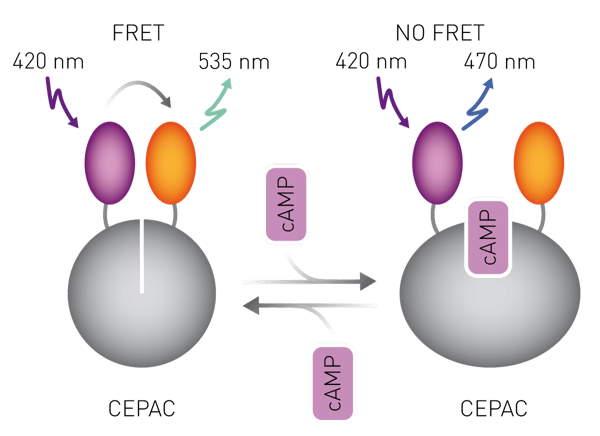
Introduction
Residues of human pharmaceuticals, such as β-blockers and their metabolites, are increasingly found in effluent wastewater of treatment plants (WWTP) all over Europe. β-blockers antagonize β-adrenergic receptors and thereby control hypertension and cardiac arrhythmias. The amino acid sequence of the target, the β1-adrenergic receptor, is evolutionarily highly conserved among vertebrates. Thus, it appears likely that organisms expressing a protein similar in function to the human β1-adrenergic receptor, also physiologically respond to β-blockers upon exposure. For risk assessment, one has to know the extent to which aquatic organisms are exposed to β-blockers as well as metabolites with the same mode of action (MOA). Therefore, we developed and validated a cell-based MOA assay, by which the total β-blocker activity in a waste-water sample enriched by solid-phase extraction (SPE) can be assessed. With our assay we are able to measure the total β-blocker activity in wastewater effluents as equivalents of the lead substance metoprolol (MetEQ).
Assay Principle
Materials & Methods
- CHO cells (DSMZ-No. ACC-110)
- Microplate (96-well, black bottom, NUNC)
- Metoprolol (Sigma)
- Isoproterenol (Sigma)
- Plasmids (SIZ Zellkulturtechnik Mannheim)
- CLARIOstar® microplate reader (BMG LABTECH)
Experimental Procedure
CHO cells were transfected with the genetically encoded fluorescent CEPAC (cAMP detecting FRET biosensor mCerulean - Epac (exchange protein directly activated by cAMP) - mCitrine) cAMP FRET sensor using FUGENE-HD (Promega). 24h after transfection, cells were selected and expanded under G418. The resulting CEPAC mixed cell clone was supertransfected with the human β1-adrenergic receptor gene. 24h after transfection, cells were selected and expanded under G418 and hygromycin. Upon induction of transgene expression via doxycycline withdrawal from the culture medium, subclones were screened for β1-adrenergic receptor activity using the agonist isoproterenol: Upon receptor activation the intracellular cAMP level increases (Fig. 1) and consequently the emission fluorescence ratio 470nm/535nm increases when excited at 420nm (Fig. 2).
In the presence of β-blockers, less cAMP is produced leading to a concentration-dependent reduction of the 470/535 ratio. The CEPAC-β1-adrenergic receptor sensor cell line was used to assay for β-blocker activities in wastewater samples. Measurements were done with the CLARIOstar microplate reader (BMG Labtech). Activities were measured in metoprolol equivalents (MetEQ).
Instrument settings
|
Optic settings |
Fluorescence intensity, well scan |
|
|
topic |
||
|
No. of multichromatic |
2 |
|
|
Monochromator Settings |
Cerulean Excitation: 420-15 Dichroic: 443.8 Emission: 470-20 Gain: 2100 |
|
|
Citrine Excitation: 420-15 Dichroic: 476.2 Emission: 535-20 Gain: 2200 |
||
|
Focal height |
4.9 mm |
|
|
Well scan |
Matrix scan |
Dimension: 4x4 mm |
|
Kinetic setting (script Wizard) |
No. of flashes per point |
8 |
|
No. of cycles |
17 |
|
|
Cycle time |
195 s |
|
|
Injection |
Injection time |
4th cycle |
|
Injected volume |
25 µL |
|
|
Pump speed |
430 µl/s |
|
Results & Discussion
Using CHO cells expressing both, the β1-adrenergic receptor and CEPAC, β-blocker activity was measured in complex mixtures such as WWTP effluents in SPE-enriched samples (Fig. 3).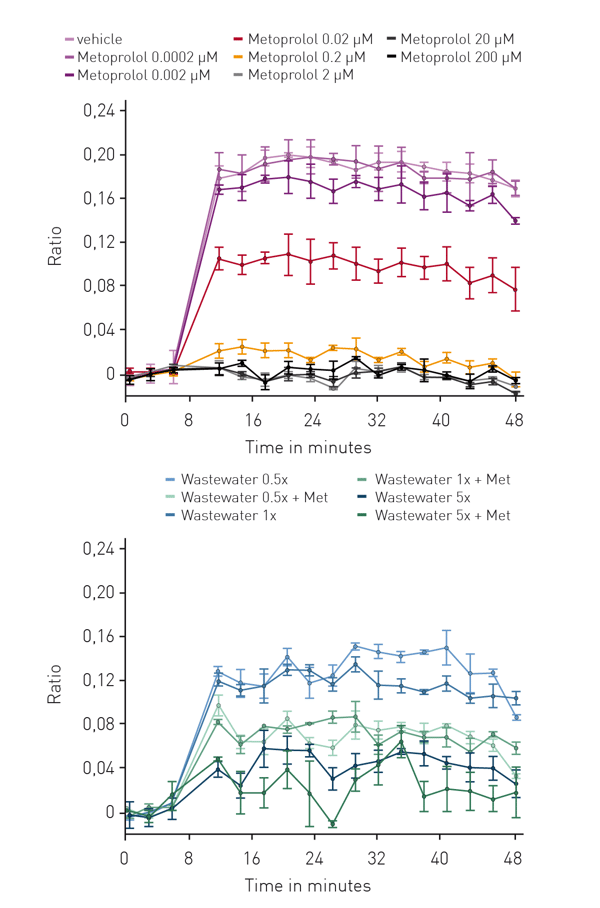
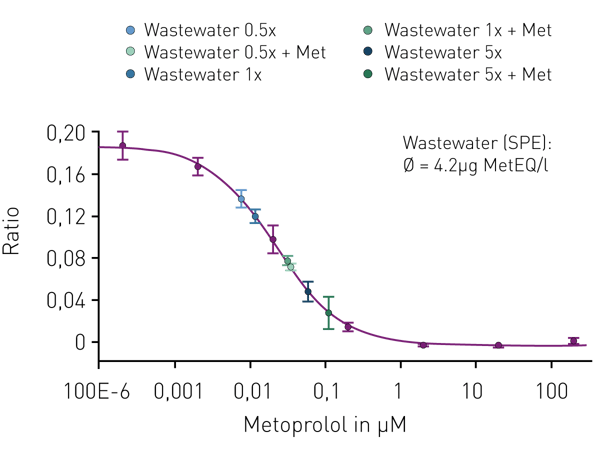 The assay was validated with the lead substance metoprolol. The β-blocker concentration of the assayed wastewater sample was found to be 4.2µg/l MetEQ (Fig. 4). Corresponding metoprolol concentration determined by chemical analysis (LC-MS/MS) was 1.2µg/l. The higher metoprolol activity measured by the in vitro assay compared to the metoprolol concentration determined by chemical analysis is explained by the presence of further β-blockers. A chemical analysis of selected pharmaceutical compounds determined additional β-blockers like bisoprolol, propranolol and atenolol in the wastewater sample (TZW Karlsruhe, data not shown)
The assay was validated with the lead substance metoprolol. The β-blocker concentration of the assayed wastewater sample was found to be 4.2µg/l MetEQ (Fig. 4). Corresponding metoprolol concentration determined by chemical analysis (LC-MS/MS) was 1.2µg/l. The higher metoprolol activity measured by the in vitro assay compared to the metoprolol concentration determined by chemical analysis is explained by the presence of further β-blockers. A chemical analysis of selected pharmaceutical compounds determined additional β-blockers like bisoprolol, propranolol and atenolol in the wastewater sample (TZW Karlsruhe, data not shown)
Conclusion
The MOA-based β-blocker assay in living cells measures total β-blocker activities in complex mixtures such as WWTP effluents as equivalents of the lead substance metoprolol (MetEQ). The assay is suitable for use as a standard method and routine employment in large-scale monitoring of WWTP effluents and the aquatic environment they are discharged into. The CLARIOstar microplate reader supports this since it provides highly stable and reliable results as well as the required robustness for continuous use.
References
- Bernhard et al. (2017) Two novel real time cell-based assays quantify beta-blocker and NSAID specific effects in effluents of municipal wastewater treatment plants, Water Research Vol. 115, pp. 74-83, May 15, 2017.