Introduction
Post-translational modification (PTM) is an essential step in cellular biology. Specific addition of residues or cleavage of existing molecular bonds are used to activate enzymes or protein-pro-forms and can even promote protein degradation. While PTM comprises a vast range of residues and cleavage sites, the phosphorylation of amino acid side chains is by far the most common modification. The understanding of protein phosphorylation is an important step, both for basic and disease-related research1,2. THUNDER™ offers a TR-FRET-based high-throughput immunoassay platform designed to enable simple, rapid, sensitive, robust, and cost-effective measurement of endogenously expressed phosphorylated proteins in whole-cell lysates3.
In this application note, we describe the validation of ERK1/2, p38αβγ and STAT3 THUNDER™ cellular kinase assays on the CLARIOstar Plus multi-mode plate reader.
Assay Principle
THUNDER™ cellular assays are homogeneous sandwich immunoassays (Figure 1) based on an enhanced TR-FRET technology developed by Bioauxilium Research. One antibody is labeled with a long lifetime Europium chelate donor (Eu-Ab1) and the second with a far-red acceptor fluorophore (FR-Ab2). Upon excitation of the Europium chelate at 320 or 340 nm, energy is transferred from the donor to the acceptor fluorophore only if the target protein is phosphorylated. The emission by the acceptor of a long-lived TR-FRET signal at 665 nm is measured after a time delay and is proportional to the concentration of phosphorylated protein in the cell lysate.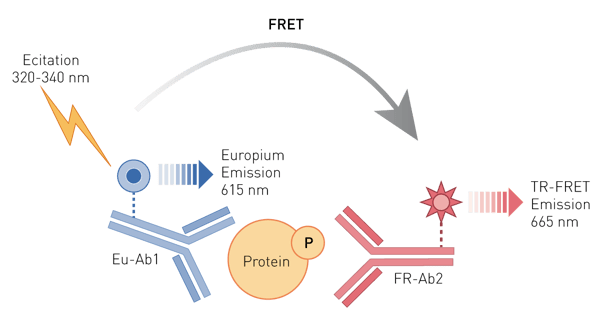
Materials and Methods
- 96-well culture plates (Costar®) and 384-well, white low-volume plates (PerkinElmer®)
- HEK293 and HeLa cells (ATCC®)
- Epidermal growth factor (EGF; PeproTech®), Interferon-alpha (INFα; ProSpec) and Anisomycin (Cayman Chemical)
- KIT-ERKP-100, KITP38αβγP-100 and KIT-STAT3P-100 assay kits (Bioauxilium Research)
- CLARIOstar Plus microplate reader
Experimental Procedure
Protocols were conducted as per Bioauxilium’s recommendations4,5,6. Cells were cultured overnight in 96-well tissue culture-treated plates. Following treatment, the media was removed and cells were lysed with the specific 1X Lysis Buffer supplemented with the Phosphatase Inhibitor Cocktail, both included in the kit. After 15-30-min incubation at room temperature (RT) on an orbital shaker (400 rpm), lysates (15 µL) were transferred to 384-well white assay plates followed by the addition of the Antibody Detection Mix (5 µL) for detection of the target protein. The plates were incubated at RT for the appropriate time and read on the CLARIOstar Plus using the settings recommended for THUNDER™. Data were expressed as (665/620) * 1,000 and are the mean ± SD of three wells per point.
Instrument Settings
| Optic settings |
Time-resolved fluorescence, plate mode endpoint | |
| Filters | Ex TR Dichroic: LP TR Em 1: 665-10 Em 2: 620-10 |
|
| Gain | 2300 | |
| Integration time | Delay: 60 µsec, Time: 400 µs | |
| General settings | Number of flashes 200 | |
| Settling time 0.1 s | ||
Results & Discussion
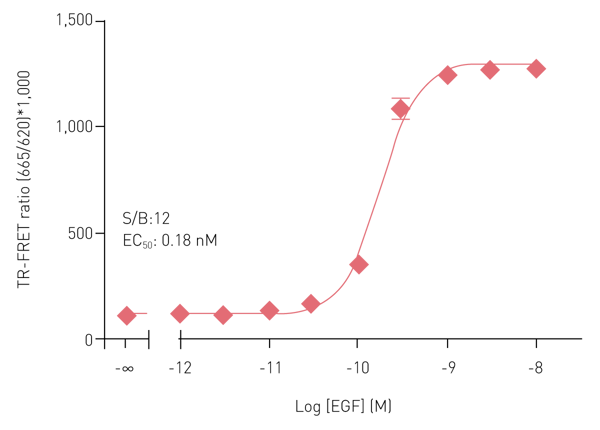
Figure 2 shows a concentration-response curve obtained with the Phospho-ERK1/2 (T202/Y204) assay kit. Treatment of HEK293 cells (50,000 cells/well) with EGF for 10 min at RT induced the phosphorylation of ERK1/2 at T202/Y204. The S/B ratios and EC50 values were consistent with the expected values.4
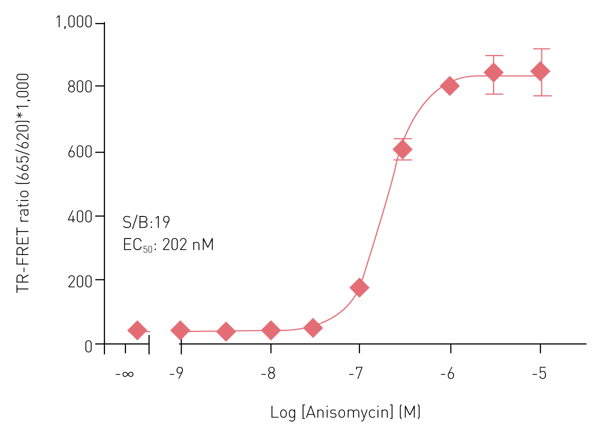
Figure 3 summarizes results obtained with the Phospho-p38αβγ (T180/Y182) assay kit. Incubation of HeLa cells (50,000 cells/well) with Anisomycin for 60 min at 37°C stimulates phosphorylation of p38αβγ at T180/Y182. The assay shows a very high S/B ratio and the expected EC505.
Results obtained with the Phospho-STAT3 (Y705) assay kit are summarized in Figure 4. HeLa cells (40,000 cells/well) treated with Interferon-alpha (IFNα) for 20 min at RT show the anticipated concentration-dependent activation of phospho-STAT3 at Y705, with a high S/B ratio and the expected EC50 value6.
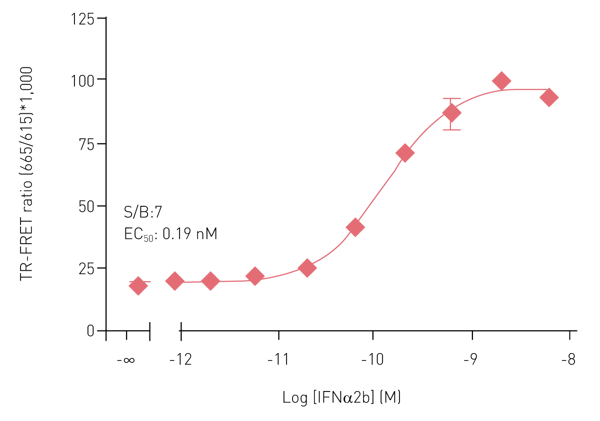 Overall, data were similar to those obtained with other TR-FRET enabled readers, demonstrating that the CLARIOstar Plus is suitable for performing THUNDER™ cellular assays. In addition, results obtained using different instrument settings (gain, delay and integration time) recommended for other TR-FRET technologies had no significant impact on the overall THUNDER™ assay performance (data not shown), further highlighting the robustness of THUNDER™ cellular kinase assays.
Overall, data were similar to those obtained with other TR-FRET enabled readers, demonstrating that the CLARIOstar Plus is suitable for performing THUNDER™ cellular assays. In addition, results obtained using different instrument settings (gain, delay and integration time) recommended for other TR-FRET technologies had no significant impact on the overall THUNDER™ assay performance (data not shown), further highlighting the robustness of THUNDER™ cellular kinase assays.
Conclusion
The current data validate the compatibility of the CLARIOstar Plus for THUNDER™ cellular kinase assays. All assays exhibited high signal levels and S/B ratios, broad dynamic ranges, and the expected potency. THUNDER™ cellular assays run on the CLARIOstar Plus are well-suitable for both basic research and drug discovery applications and offer a straightforward approach for the determination of protein phosphorylation levels in cell lysates.
References
- Ardito, F., et al. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int J Mol Med, 2017, 40(2), 271-280.
- Singh, V., et al. Phosphorylation: Implications in Cancer. Protein J, 2017, 36(1), 1-6.
- bioauxilium.com/about-tr-fret/
- bioauxilium.com/product/phospho-erk1-2-t202-y204/
- bioauxilium.com/product/phospho-p38-mapk-t180-y182/
- bioauxilium.com/product/phospho-stat3-y705/