
SPECTROstar Nano
Absorbance plate reader with cuvette port
The LAL assay ensures that sterile pharmaceutical products and medical devices are safe for human use. This test can be run efficiently and in high throughput on a plate reader.
 Dr Tobias Pusterla
Dr Tobias Pusterla
Parenteral (intravenous) drugs have to meet specific safety standards. One of these is the absence of endotoxins, bacterial structural components that are toxic for humans and animals, and that cause pyrogenic responses (fever) by the organism’s immune system. The same standard applies as well to medical devices such as disposable equipment and implants.
Although there are several methods to test for bacterial endotoxin presence, the Limulus Amebocyte Lysate (LAL) assay remains the gold standard. The LAL assay is used to ensure the safety of parenteral drugs, biologicals (e.g., insulin) and medical implants. In addition, it is used in environmental air and water testing.
Based on appearances you probably would not expect this creature to hold the key to ensuring the safety of a multitude of medical devices and drugs that are currently on the market. The horseshoe crab (Limulus polyphemus) not only looks bizarre (fig.1), it is quite literally from another time – 450 million years ago! It definitely earns the description as a ‘living fossil’.
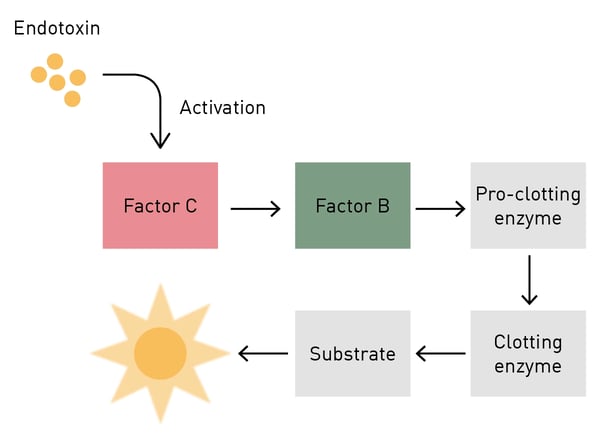
 To understand the importance of the limulus in medicine you must look beneath the surface, specifically at its blood – beyond the fact that it is light blue in colour. The medical interest in limulus blood is based on the presence of cells called amebocytes. These cells are vital in the survival of the horseshoe crab as they serve as the key to its innate immunity against fungi and Gram-negative bacteria. Amebocytes contain granules with a clotting factor called coagulogen. When the amebocytes come in contact with bacterial endotoxin which is found in the cell walls of these intruders, coagulogen is released and an enzymatic cascade is triggered, eventually resulting in the coagulation (clotting or gelling), immobilization and neutralization of the pathogen. The starting point of the clotting reaction is clotting factor C.1
To understand the importance of the limulus in medicine you must look beneath the surface, specifically at its blood – beyond the fact that it is light blue in colour. The medical interest in limulus blood is based on the presence of cells called amebocytes. These cells are vital in the survival of the horseshoe crab as they serve as the key to its innate immunity against fungi and Gram-negative bacteria. Amebocytes contain granules with a clotting factor called coagulogen. When the amebocytes come in contact with bacterial endotoxin which is found in the cell walls of these intruders, coagulogen is released and an enzymatic cascade is triggered, eventually resulting in the coagulation (clotting or gelling), immobilization and neutralization of the pathogen. The starting point of the clotting reaction is clotting factor C.1
The ability of amebocytes to produce a visible response to the presence of bacterial endotoxin is the basis for the LAL test. The LAL assay is based on an extract of isolated amebocytes (LAL) from the blood of the Limulus polyphemus. 1 The TAL (tachypleus amebocyte lysate) assay is the LAL twin assay and is mainly used in Asia, based on the local availability of another horseshoe crab (Tachypleus gigas or tridentatus).2 There are three basic methodologies for the LAL test: gel clot, chromogenic and turbidimetric.
Gel clot LAL test
The gel clot LAL method was the initial and is the simplest form of LAL assay. It is a qualitative test in which the formation of a clot or gel at the bottom of the sample tube is examined by eye and indicates the presence of endotoxin in a sample.
Chromogenic LAL assay
The chromogenic LAL assay is a quantitative test that produces a yellow colour (chromogenic) as the result of an enzymatic reaction triggered by the activation of limulus clotting factor C by the presence of bacterial endotoxin in a sample (fig.2). Within a kit specific range, the colour intensity is directly proportional to the quantity of contaminants in the sample: the more endotoxin, the stronger yellow the solution will become. The chromogenic LAL reaction can be quantified (usually using endotoxin units (EU) per ml or per sample) by absorbance detection using a spectrophotometer or an absorbance microplate reader.
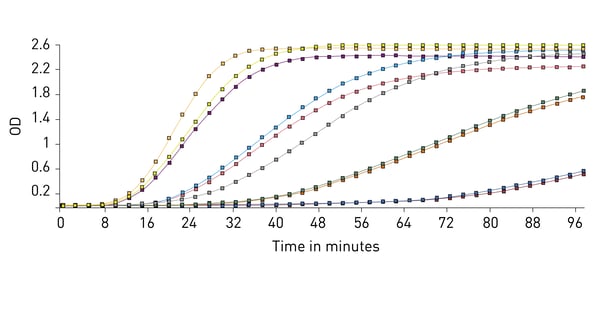 The chromogenic LAL assay is typically used to test small volumes of substances such as antibiotics, vaccines, parenteral drugs, etc. Especially when quantified on a microplate reader, the advantages of the chromogenic LAL assay are the possibility to increase efficiency and throughput and to automate the process.
The chromogenic LAL assay is typically used to test small volumes of substances such as antibiotics, vaccines, parenteral drugs, etc. Especially when quantified on a microplate reader, the advantages of the chromogenic LAL assay are the possibility to increase efficiency and throughput and to automate the process.
Turbidimetric LAL assay
The turbidimetric variant of the LAL test is typically used when samples have an intrinsic colour that could interfere with the yellow readout of the chromogenic LAL test. In this case, LAL clotting is exploited to produce particulates that make the solution become turbid. The turbidity of the sample is then quantified and is not affected by the colour of the sample.
Both chromogenic and turbidimetric LAL assays can be quantified on a plate reader. These approaches are quite straightforward and only require an absorbance microplate reader to detect either the onset of the yellow colour of the chromogenic LAL reaction at 405-410 nm wavelength, or the changes in turbidity for the LAL turbidimetry assay at a wavelength of 340 nm.
All BMG LABTECH readers that are equipped for absorbance are up to the task of reading the LAL assay. These include the SPECTROstar Nano, Omega series, VANTAstar, CLARIOstar Plus and PHERAstar FSX.
MARS data analysis software can also be used to perform kinetic calculations. For this test comparing the time it takes each sample to reach a certain OD threshold is important and is used to compare samples of known concentrations and create a standard curve that is used to calculate unknowns.
The application note “Lonza’s kinetic kit for endotoxin detection using BMG LABTECH’s microplate reader and MARS data analysis“ provides an example of how a chromogenic LAL assay is run on a microplate reader.
Unfortunately, LAL production is dependent on a rather crude process which involves the capture of horseshoe crabs (over 500,000 in 2015 alone) so that some blood can be collected (fig.4). The blood draw volume can be from 50 to 400 mL dependent on sex and size. The blood is centrifuged to concentrate the amebocytes which are then lysed to release the coagulation proteins, the very LAL. Although the horseshoe crabs are able to be released after bleeding, a certain number (10 – 30 %) are known to perish during the transport and/or blood extraction process. Further complications may occur leading to death after release which would not be directly associated with LAL test production.
 Another issue is that limulus capture is occurring during mating as this is the time when they come ashore. It is unclear what effect the collection has on the reproductive capacity, especially among captured females. What is clear is that the number of horseshoe crabs is in decline and human collection for LAL testing is certainly a contributing factor.
Another issue is that limulus capture is occurring during mating as this is the time when they come ashore. It is unclear what effect the collection has on the reproductive capacity, especially among captured females. What is clear is that the number of horseshoe crabs is in decline and human collection for LAL testing is certainly a contributing factor.
Efforts are underway to ensure better practices in the collection of the crabs including identifying the best possible mechanisms of transport, understanding the best temperatures to use and limiting the time they are out of water. Limiting the capture of the females of the species has also been proposed as a way to promote higher reproductive capacity. Furthermore, a better understanding of the impact of the bleeding process must be achieved. This needs to include how much blood can be safely drawn from an individual of a given size. Unfortunately, ‘ranching’ of horseshoe crabs has thus far proved extremely unsuccessful so capture of wild crabs will continue so long as there is demand.
The need for LAL assays shows no signs of decreasing and in fact there are fears that demand may increase to keep up with the latest medical advances. Moreover, ethical and conservation issues have been raised as the LAL assay depends on the exploitation of wild animals. All these concerns together raised the necessity to develop “synthetic” LAL alternatives.
Thus far, there has been resistance to adopting any of the LAL alternative methods to detect endotoxin. This is understandable for the fact that approval of any process used in safety testing is difficult, but for those seeking alternatives they are definitely available.
Recombinant factor C
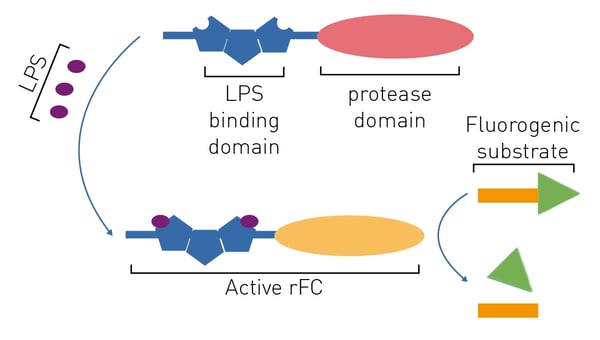
A chromogenic LAL substitute based on recombinant proteins is commercially available (e.g., the PyroGeneTM assay). Limulus clotting factor C is the initiating protease of the limulus coagulation cascade upon exposure to endotoxin. The recombinant limulus clotting factor C protein (rFC) has been cloned from the Singapore horseshoe crab.4 Knowledge of the cleavage site in rFC´s substrate, factor B, enabled the production of assays based on recombinant proteins.
The rFC test is promising because it is more sensitive than the LAL and has a better specificity for endotoxin.3 The rFC assay is a quantitative test that uses a fluorogenic substrate (excitation at 380 nm, emission at 440 nm) and hence requires a fluorescence microplate reader to be quantified (fig. 5). This is further discussed in the application note “Faster PyroGene™ Detection of Endotoxin using Enhanced Dynamic Range on the CLARIOstar Plus”. The readout by means of fluorescence intensity also accounts for the higher sensitivity of this assay.
Monocyte Activation Test
Another LAL alternative is the monocyte activation test (MAT). MAT is an in vitro cell-based alternative way to test for endotoxins. It measures the endotoxin-driven release of cytokines from monocytes and is based on their innate immune response to pathogens. For this purpose, monocytes or Peripheral Blood Mononuclear Cells (PBMC) are exposed to the test sample. After an incubation time, the supernatant is tested for the presence of inflammatory cytokines (e.g., interleukin-6) typically by ELISA. While this approach is more laborious than a LAL assay, it also detects Gram-positive bacteria, yeasts, moulds and viruses instead of only endotoxins from Gram-negative bacteria as the LAL test does.
Absorbance plate reader with cuvette port
Powerful and most sensitive HTS plate reader
Most flexible Plate Reader for Assay Development
Upgradeable single and multi-mode microplate reader series
Flexible microplate reader with simplified workflows
ELISAs are a popular tool to detect or measure biological molecules in the life sciences. Find out how microplate readers can be used to advance research using immunoassays.
Antimicrobial resistance is a formidable problem across the world. Find out how microplate readers can help tackle resistant microbes.
Next generation sequencing (NGS) technologies for DNA or RNA have made tremendous progress in recent years. Find out how microplate readers can advance the quality control of nucleic acids to facilitate NGS.
Light scattering offers distinct advantages for scientists interested in immunology. Find out how the NEPHELOstar Plus is used for high-throughput immunological tests.
This article on gene delivery presents methods being explored to deliver correcting DNA sections to restore target gene expression in patients.